Novavax COVID-19 Vaccine Gets FDA Nod, With Unusual Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
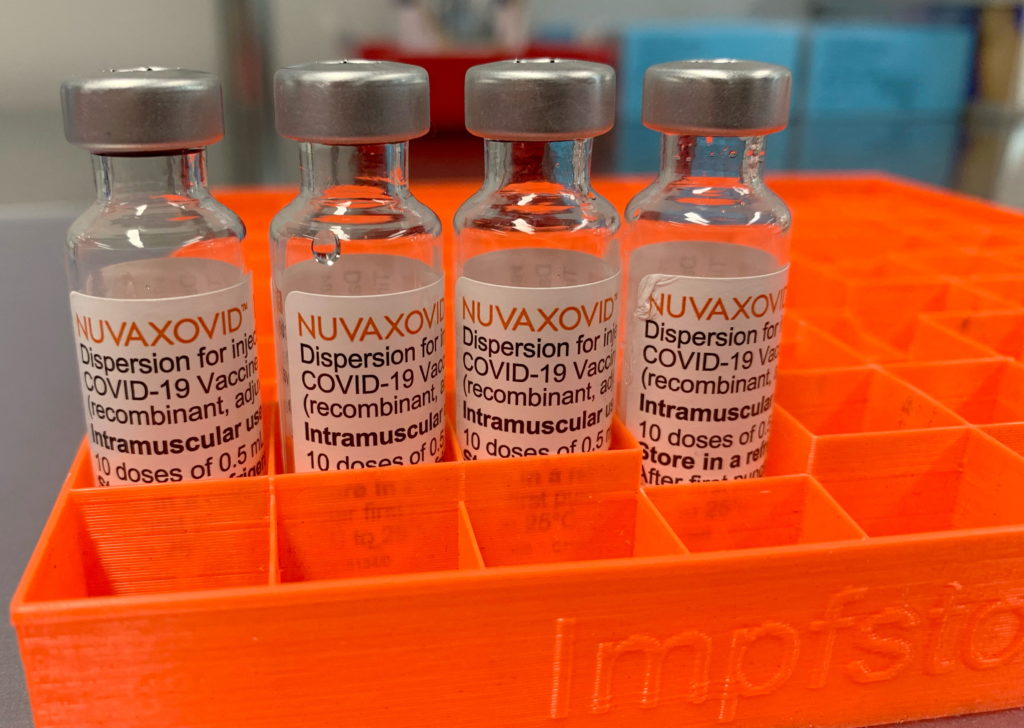
Novavax COVID-19 Vaccine Gets FDA Nod, but with Unusual Usage Restrictions
The FDA has finally granted emergency use authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, offering a new option for those seeking vaccination. However, the authorization comes with unusual and noteworthy restrictions, sparking discussion among health experts and the public. This development marks a significant shift in the COVID-19 vaccine landscape, but understanding the nuances of the FDA's decision is crucial.
A Traditional Approach in a Changing Landscape
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax's Nuvaxovid utilizes a more traditional protein subunit technology. This approach involves using harmless pieces of the virus to trigger an immune response, a method familiar from other established vaccines. This familiarity might appeal to some individuals hesitant about newer technologies. The vaccine's protein-based approach has been touted as potentially less prone to some of the side effects seen with other COVID-19 vaccines, although further long-term data is needed to confirm this.
The Unexpected Restrictions: Why the Cautious Approach?
While the approval is undoubtedly positive news, the FDA's decision comes with a caveat: Nuvaxovid's authorization is specifically for adults aged 18 years and older. This is unlike the other authorized vaccines which have expanded approvals for adolescents and even younger children. This restricted age range raises questions about the vaccine's efficacy and safety profile in younger populations.
Furthermore, the FDA's authorization emphasizes that Nuvaxovid should only be used in individuals who have not received any other COVID-19 vaccine. This is a significant departure from the flexibility offered by other vaccines, where individuals can choose different manufacturers for primary or booster shots. This limited usage significantly restricts the potential reach of the Novavax vaccine.
Possible Reasons Behind the Restrictions
The reasons behind these restrictions aren't explicitly detailed, but experts speculate that they might stem from several factors:
- Limited Clinical Trial Data: The FDA may have required more extensive data on the vaccine's safety and efficacy in younger age groups and in individuals already exposed to other COVID-19 vaccines. Such data may be still under collection and analysis.
- Manufacturing Capacity: Novavax has experienced production delays. Focusing the initial rollout on a specific population may allow for better allocation of limited vaccine supplies.
- Competitive Landscape: With other effective COVID-19 vaccines already widely available, the FDA's cautious approach might reflect a more measured integration of Nuvaxovid into the existing vaccination strategy.
What This Means for the Future of COVID-19 Vaccination
The FDA's authorization of Novavax's vaccine, albeit with restrictions, provides an additional tool in the fight against COVID-19. However, the unusual limitations raise questions about its widespread adoption. The limited usage guidelines could constrain its impact, particularly given the already high vaccination rates in many countries. Further research and data collection will be critical in determining whether these restrictions will be lifted in the future. This development highlights the ongoing need for diverse vaccine options and continued vigilance in monitoring vaccine safety and efficacy.
Call to Action: Stay informed about the latest COVID-19 vaccine updates from reliable sources like the and . Consult with your healthcare provider to discuss the best vaccination strategy for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, With Unusual Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Evaluating Backup Quarterbacks Who Could Spearhead A 2024 Nfl Playoff Run
May 20, 2025
Evaluating Backup Quarterbacks Who Could Spearhead A 2024 Nfl Playoff Run
May 20, 2025 -
 Novavax Covid 19 Vaccine Fda Approval Comes With Unusual Usage Constraints
May 20, 2025
Novavax Covid 19 Vaccine Fda Approval Comes With Unusual Usage Constraints
May 20, 2025 -
 Japan Accepts Lower Us Tariffs Following Trade Negotiation Stalemate
May 20, 2025
Japan Accepts Lower Us Tariffs Following Trade Negotiation Stalemate
May 20, 2025 -
 Free Mlb Home Run Prop Bets For May 17 Top Picks And Analysis
May 20, 2025
Free Mlb Home Run Prop Bets For May 17 Top Picks And Analysis
May 20, 2025 -
 Can These Backup Quarterbacks Lead An Nfl Team To A Playoff Victory In 2024
May 20, 2025
Can These Backup Quarterbacks Lead An Nfl Team To A Playoff Victory In 2024
May 20, 2025
