Novavax COVID-19 Vaccine: FDA Approval Comes With Unusual Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval Comes with Unusual Usage Constraints
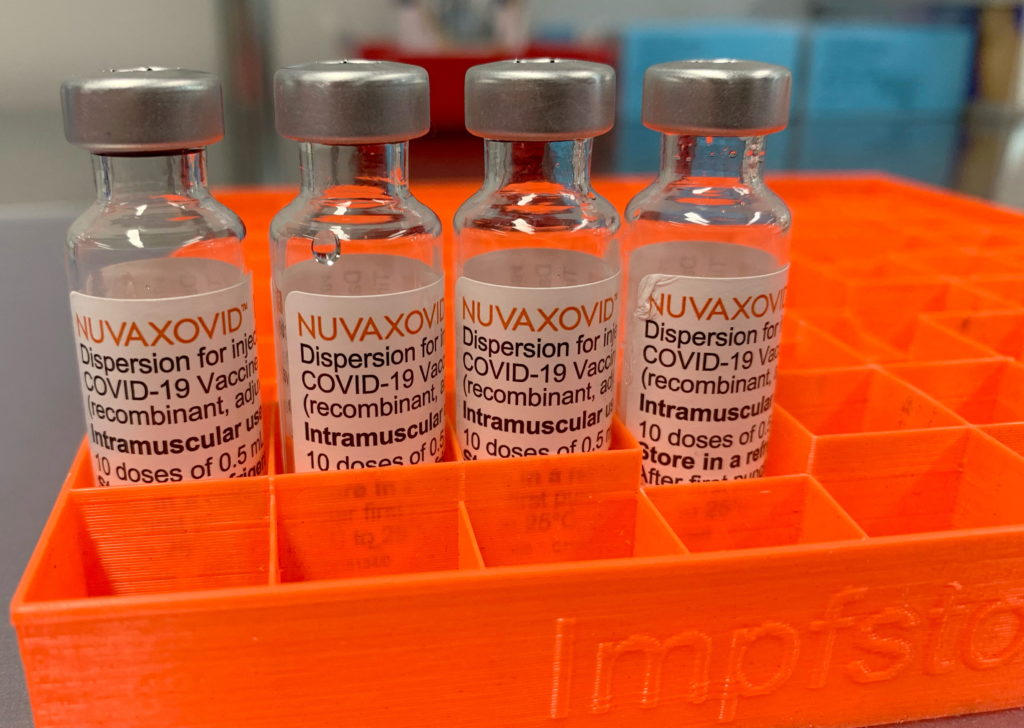
The Novavax COVID-19 vaccine, Nuvaxovid, finally received FDA approval in July 2023, marking a significant milestone for the pharmaceutical company. However, this approval comes with some unusual and noteworthy usage constraints, raising questions about its market impact and public health implications. Unlike other widely-used COVID-19 vaccines, Nuvaxovid's rollout faces unique hurdles. Let's delve into the details.
Limited Target Audience & Conditional Approval:
The FDA's approval wasn't a blanket endorsement. Instead, it's a conditional approval, primarily targeting adults aged 18 years and older. This differs significantly from vaccines like Pfizer-BioNTech and Moderna, authorized for use in younger age groups. This limited target audience immediately restricts the vaccine's potential reach and market penetration. The conditional approval also indicates that ongoing monitoring and further data collection are required to fully assess its long-term safety and efficacy. This cautious approach reflects the FDA's commitment to rigorous safety standards, even after the initial emergency use authorization (EUA) phase.
Why the Conditional Approval and Usage Constraints?
Several factors contribute to the unusual constraints surrounding the Novavax vaccine's approval. While the vaccine demonstrated efficacy in clinical trials, the data wasn't as robust or comprehensive as that supporting other already-approved vaccines. Furthermore, the emergence of newer COVID-19 variants and the changing epidemiological landscape influenced the FDA's decision. The agency likely wants to observe the vaccine's performance in real-world scenarios before expanding its authorization to broader age groups.
Potential Impact on Vaccination Campaigns:
The restricted usage limits the vaccine's role in broader public health initiatives. With other readily available vaccines already offering broader age-group coverage and established safety profiles, the addition of Nuvaxovid, with its limitations, might not significantly alter existing vaccination strategies. This could lead to challenges in promoting and distributing the vaccine, especially when competing against well-established alternatives.
The Role of Vaccine Hesitancy:
Some individuals express hesitancy towards mRNA vaccines, potentially viewing the Novavax vaccine – a protein-subunit vaccine – as a more acceptable alternative. However, the limited availability and usage restrictions could inadvertently dampen this potential demand. The complex interplay between vaccine hesitancy, limited access, and the availability of other options creates a challenging landscape for the successful integration of Nuvaxovid into vaccination campaigns.
What Does the Future Hold for Nuvaxovid?
The future of the Novavax COVID-19 vaccine hinges on several factors. Continued monitoring of its safety and efficacy, further clinical trials exploring its use in different age groups, and evolving public health needs will all play crucial roles. The FDA's conditional approval provides a pathway for potential expansion, but the journey to broader acceptance and wider usage will depend on overcoming the current limitations and demonstrating its sustained value in a changing COVID-19 landscape.
Key Takeaways:
- Conditional Approval: The FDA approval is conditional, requiring ongoing monitoring and data collection.
- Limited Target Audience: Currently approved only for adults 18 years and older.
- Impact on Vaccination Strategies: Its limited usage may not significantly alter existing vaccination campaigns.
- Vaccine Hesitancy Factor: While appealing to some vaccine-hesitant individuals, its limitations might hinder widespread adoption.
- Uncertain Future: The long-term success of Nuvaxovid depends on ongoing data collection and addressing current limitations.
This situation highlights the complexities of vaccine approval and distribution, emphasizing the need for careful consideration of both scientific data and public health implications. Only time will tell whether Novavax can overcome these challenges and secure a significant place in the ongoing fight against COVID-19.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval Comes With Unusual Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Balis Tourism Plea Prioritizing Safety And Responsible Tourist Behavior
May 20, 2025
Balis Tourism Plea Prioritizing Safety And Responsible Tourist Behavior
May 20, 2025 -
 Pet Cremation Scandal Fuels Calls For Stronger Consumer Protections
May 20, 2025
Pet Cremation Scandal Fuels Calls For Stronger Consumer Protections
May 20, 2025 -
 Heated Wnba Debut Caitlin Clarks Triple Double And Confrontation With Angel Reese
May 20, 2025
Heated Wnba Debut Caitlin Clarks Triple Double And Confrontation With Angel Reese
May 20, 2025 -
 Otra Vez En Vivo Duo De Yahir Y Victor Garcia En Juego De Voces 2025
May 20, 2025
Otra Vez En Vivo Duo De Yahir Y Victor Garcia En Juego De Voces 2025
May 20, 2025 -
 Jon Jones Vs Ufc The Aspinall Injury Information Controversy
May 20, 2025
Jon Jones Vs Ufc The Aspinall Injury Information Controversy
May 20, 2025
