Novavax COVID-19 Vaccine: FDA Approval, But With Significant Caveats
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval, but with Significant Caveats
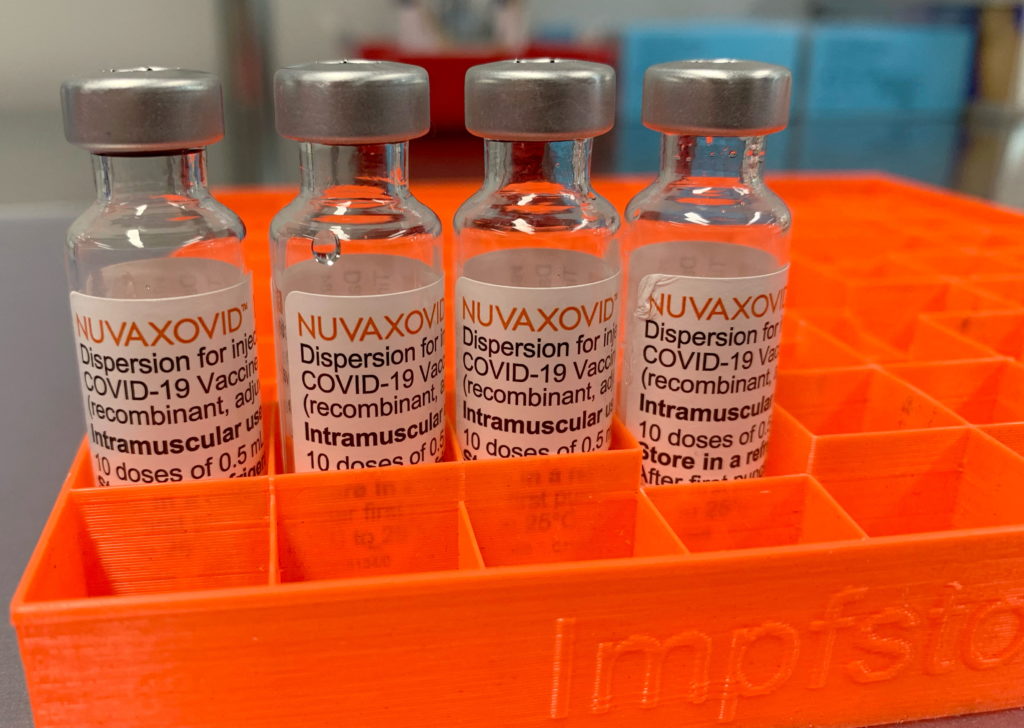
The Novavax COVID-19 vaccine, Nuvaxovid, finally received FDA approval in July 2023, marking a significant milestone in the fight against the pandemic. However, this approval comes with considerable caveats that temper the initial celebratory mood. While offering a protein-subunit alternative to mRNA vaccines, Nuvaxovid's path to widespread adoption faces several hurdles. This article delves into the FDA's decision, highlighting the crucial limitations and implications for public health.
A Protein-Subunit Approach: A Different Technology
Unlike the Pfizer-BioNTech and Moderna vaccines which utilize mRNA technology, Novavax's vaccine employs a more traditional protein-subunit approach. This method uses lab-grown pieces of the virus's spike protein to trigger an immune response. This difference has been a source of both hope and hesitation. Many individuals hesitant about mRNA technology viewed Novavax as a potentially safer alternative. However, the reality of its approval and subsequent rollout paints a more complex picture.
FDA Approval: A Qualified Victory
The FDA's approval was not a blanket endorsement. The agency emphasized several key caveats:
- Limited Effectiveness: Clinical trial data showed Nuvaxovid's effectiveness to be lower compared to mRNA vaccines, particularly against newer variants. This raises questions about its efficacy in the current epidemiological landscape.
- Safety Concerns: While generally well-tolerated, the vaccine has shown some side effects, including pain at the injection site, fatigue, and muscle aches. These are generally mild, but their prevalence needs to be considered.
- Logistical Challenges: The vaccine requires specific storage and handling conditions, potentially posing logistical challenges for widespread distribution, particularly in resource-limited settings. This contrasts with the more robust storage capabilities of mRNA vaccines.
Market Competition and Demand:
The approval comes at a time when COVID-19 vaccination rates have plateaued, and the demand for any new vaccine is uncertain. The prevalence of variants resistant to older vaccines also significantly impacts the potential market for Nuvaxovid. The lower efficacy against newer strains compared to updated mRNA boosters makes its role in future vaccination campaigns uncertain.
Future Implications and Research:
Further research is crucial to assess Nuvaxovid's long-term efficacy and safety. Ongoing studies are monitoring its effectiveness against emerging variants and its potential use as a booster shot. The FDA's conditional approval highlights the need for continuous monitoring and data analysis.
Conclusion: A Cautious Optimism
The FDA approval of the Novavax COVID-19 vaccine represents a step forward in offering a diverse range of vaccine options. However, the significant caveats accompanying the approval underscore the need for realistic expectations. While offering a protein-subunit alternative, its lower efficacy and logistical challenges pose significant limitations. The vaccine's future role in global vaccination strategies remains uncertain, contingent on further research and evolving epidemiological circumstances. It's crucial to stay informed about updated guidance from public health organizations like the CDC and WHO regarding COVID-19 vaccination.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein-subunit vaccine, mRNA vaccine, vaccine efficacy, vaccine safety, vaccine rollout, COVID-19 variants, public health, vaccination rates.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval, But With Significant Caveats. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Controversy Erupts Analysis Of Fever Players Post Game Comments On Angel Reese
May 21, 2025
Controversy Erupts Analysis Of Fever Players Post Game Comments On Angel Reese
May 21, 2025 -
 Record Bitcoin Etf Investments Top 5 Billion Implications For The Future
May 21, 2025
Record Bitcoin Etf Investments Top 5 Billion Implications For The Future
May 21, 2025 -
 No Oval Experience No Problem Shwartzmans Indy 500 Triumph
May 21, 2025
No Oval Experience No Problem Shwartzmans Indy 500 Triumph
May 21, 2025 -
 5 Billion Poured Into Bitcoin Etfs Understanding The Markets Directional Shift
May 21, 2025
5 Billion Poured Into Bitcoin Etfs Understanding The Markets Directional Shift
May 21, 2025 -
 Can The Celtics Stay Competitive Evaluating Their Championship Chances Post Season
May 21, 2025
Can The Celtics Stay Competitive Evaluating Their Championship Chances Post Season
May 21, 2025
