Novavax COVID-19 Vaccine Approved By FDA, But With Caveats
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Approved by FDA, But With Caveats
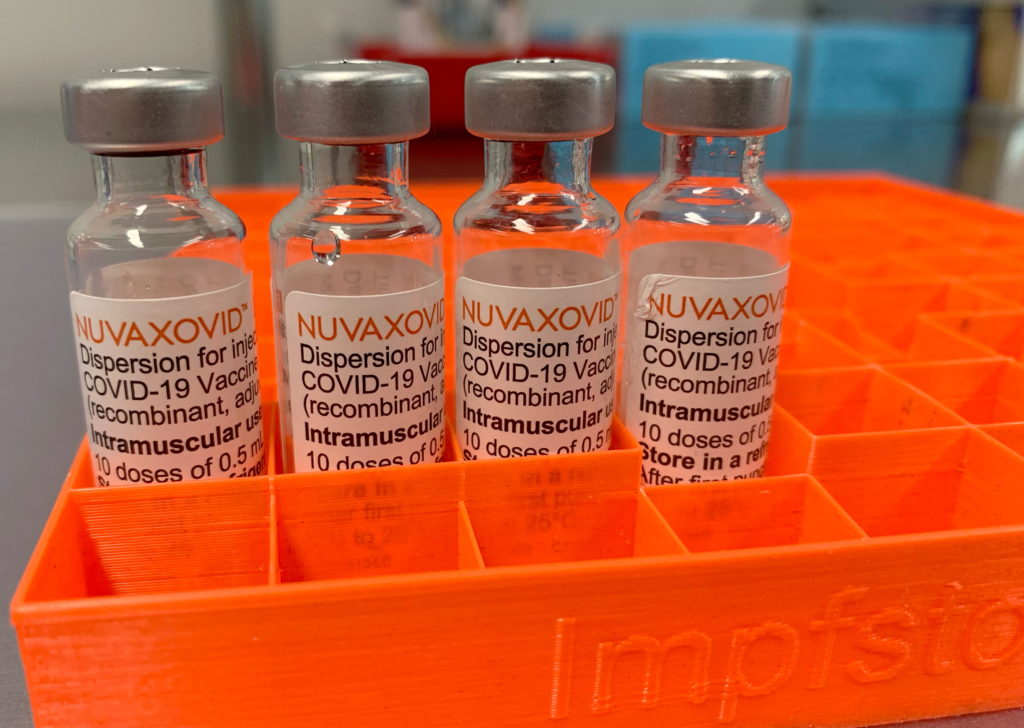
The FDA has finally granted full approval to Novavax's COVID-19 vaccine, Nuvaxovid, a move welcomed by many but tempered by significant caveats. This approval marks a significant milestone for Novavax, but its impact on the broader vaccination landscape remains uncertain. For months, the vaccine has been available under Emergency Use Authorization (EUA), but full approval brings a new level of confidence – and scrutiny.
What Does Full FDA Approval Mean?
Full approval signifies that the FDA has completed a rigorous review of all available data on Nuvaxovid's safety and efficacy. This contrasts with the EUA process, which allows for faster authorization during public health emergencies based on less complete data. Full approval typically leads to increased public trust and may influence vaccine mandates and insurance coverage policies. However, it's crucial to understand that even with full approval, ongoing monitoring for safety continues.
The Caveats: Why the Cautious Optimism?
While the approval is undoubtedly positive news, several factors temper the celebration. Firstly, the approval is specifically for individuals 18 years of age and older. This excludes a significant portion of the population, unlike some other widely available COVID-19 vaccines. Furthermore, the efficacy data presented during the approval process showed a lower efficacy rate compared to mRNA vaccines like Pfizer-BioNTech and Moderna. While still effective, this difference could impact its uptake.
Efficacy and Safety Concerns:
The FDA's assessment indicated Nuvaxovid's efficacy against COVID-19, but the specific numbers are critical to understand. While the exact figures vary based on the specific clinical trials, some studies have shown lower effectiveness compared to other vaccines, particularly against newer variants. The FDA will continue to monitor real-world data for further insights into long-term efficacy and the vaccine's effectiveness against emerging variants.
Safety remains a paramount concern. While clinical trials have demonstrated a generally acceptable safety profile, post-market surveillance is crucial to detect any rare or unforeseen adverse events. The FDA emphasizes the importance of reporting any suspected adverse reactions to the Vaccine Adverse Event Reporting System (VAERS). [Link to VAERS website]
The Role of Nuvaxovid in the Current Vaccination Landscape:
The arrival of Nuvaxovid offers an alternative for individuals who may have hesitated to receive mRNA vaccines due to concerns about their technology. This protein-subunit vaccine uses a more traditional approach, which may appeal to a segment of the population. However, its lower efficacy compared to mRNA vaccines and the limited age range for approval may restrict its widespread adoption. The availability of Nuvaxovid could potentially improve overall vaccination rates, but its actual impact remains to be seen.
The Future of Nuvaxovid:
The FDA approval is a significant step, but the success of Nuvaxovid depends on several factors including public perception, healthcare provider recommendations, and the continued evolution of the virus. Novavax will likely focus on addressing the limitations highlighted by the FDA, potentially through further research and development to improve efficacy and expand the age range for approval. The company's commitment to ongoing research will be crucial in ensuring the long-term viability of the vaccine.
Conclusion:
The FDA's full approval of the Novavax COVID-19 vaccine is a development worthy of attention, but it's important to approach the news with a balanced perspective. While it offers a valuable alternative for some, the caveats surrounding its efficacy and age limitations need careful consideration. The long-term impact of Nuvaxovid on the global vaccination effort remains to be seen. Continued monitoring and research are vital to fully understand its role in combating the ongoing COVID-19 pandemic.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Approved By FDA, But With Caveats. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Ranking Nfl Backup Quarterbacks Who Could Steal A Playoff Spot In 2024
May 21, 2025
Ranking Nfl Backup Quarterbacks Who Could Steal A Playoff Spot In 2024
May 21, 2025 -
 Two Teens Face Charges After Church Defecation Incident In Santa Rosa
May 21, 2025
Two Teens Face Charges After Church Defecation Incident In Santa Rosa
May 21, 2025 -
 2025 Indy 500 Updated Odds After Shwartzmans Stunning Pole Win
May 21, 2025
2025 Indy 500 Updated Odds After Shwartzmans Stunning Pole Win
May 21, 2025 -
 Lsus Angel Reese Faces Backlash Players Coaches React To Ncaa Tournament Controversy
May 21, 2025
Lsus Angel Reese Faces Backlash Players Coaches React To Ncaa Tournament Controversy
May 21, 2025 -
 Novavaxs Covid 19 Vaccine Gets Fda Approval Use Significantly Curtailed
May 21, 2025
Novavaxs Covid 19 Vaccine Gets Fda Approval Use Significantly Curtailed
May 21, 2025
