Novavax's COVID-19 Vaccine Gets FDA Approval, Use Significantly Curtailed
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax's COVID-19 Vaccine Gets FDA Approval, But Use Remains Significantly Limited
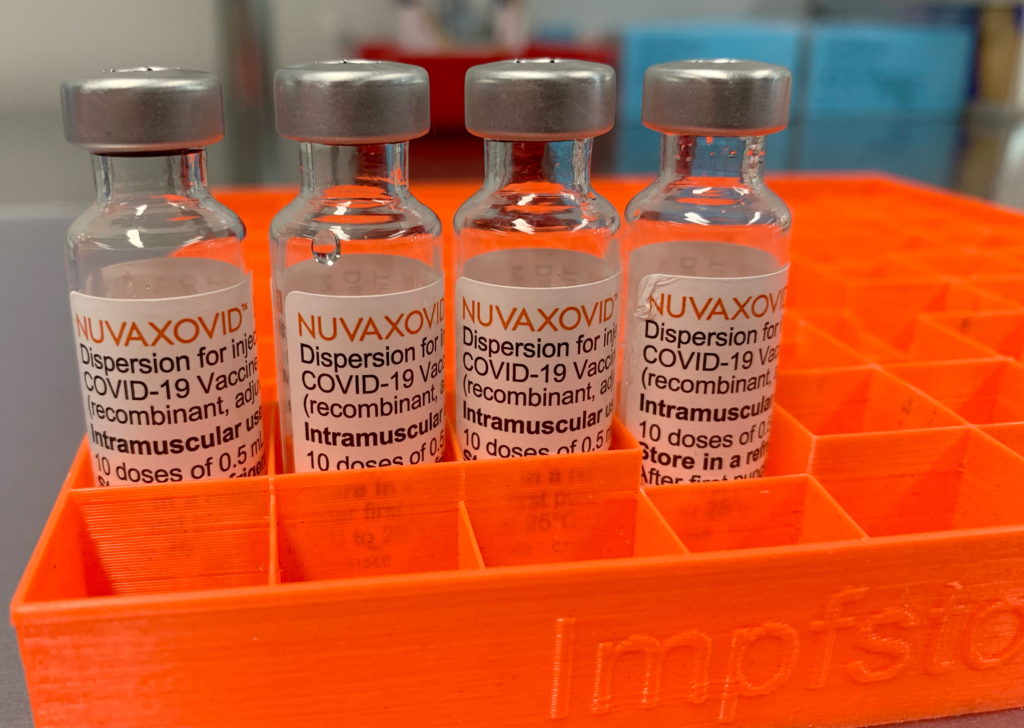
Novavax's COVID-19 vaccine, Nuvaxovid, has finally received full FDA approval, marking a significant milestone for the company. However, the news is tempered by the reality that the vaccine's use will be significantly curtailed due to the evolving landscape of the COVID-19 pandemic and the widespread availability of other, more commonly used vaccines.
The FDA's approval, announced [Insert Date], grants full licensure for Nuvaxovid for individuals 18 years of age and older. This follows the vaccine's previous Emergency Use Authorization (EUA) which allowed its use under specific circumstances. While the approval is a testament to the vaccine's safety and efficacy, the practical implications are limited.
Why the Limited Use?
Several factors contribute to the restricted application of Novavax's vaccine despite FDA approval:
- Low Demand: The demand for COVID-19 vaccines has significantly decreased since the peak of the pandemic. With readily available mRNA vaccines (like Pfizer-BioNTech and Moderna) and other established options, the need for an additional vaccine is minimal for most of the population.
- Efficacy Concerns: While Nuvaxovid demonstrated efficacy in clinical trials, its effectiveness against newer variants of concern is less pronounced compared to the updated mRNA boosters. This makes it a less attractive option for individuals seeking optimal protection.
- Logistical Challenges: The production and distribution of Nuvaxovid have faced challenges, hindering its widespread availability and contributing to its lower uptake.
- Competition: The existing vaccine market is already saturated with highly effective and widely accessible options. This makes it difficult for Nuvaxovid to gain significant market share.
What Does This Mean for the Future of Novavax?
The FDA approval is undoubtedly a positive development for Novavax, bolstering its credibility and potentially opening doors for future vaccine development. However, the company faces a significant challenge in establishing a substantial market for Nuvaxovid in the current environment. The focus may shift towards other potential vaccine candidates or exploring different avenues within the healthcare sector.
The company may need to adapt its strategies, potentially targeting specific populations or geographical regions where its vaccine might be more relevant. This might involve focusing on areas with limited access to other vaccines or where specific characteristics of Nuvaxovid (such as its protein-based platform) make it a more suitable option.
The Bigger Picture: COVID-19 Vaccination in 2024
The ongoing evolution of the COVID-19 virus and the availability of updated booster shots continue to shape the landscape of vaccination strategies. While full FDA approval is a crucial step for any vaccine, the success of Nuvaxovid will depend on factors beyond its scientific merit, including market demand, logistical capabilities, and the competitive landscape. The future of COVID-19 vaccination remains fluid and will continue to adapt to the ever-changing dynamics of the pandemic.
For more information on COVID-19 vaccines and current recommendations, consult your healthcare provider or visit the CDC website: [Link to CDC Website]
This article is for informational purposes only and does not constitute medical advice. Always consult with a qualified healthcare professional for any health concerns or before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax's COVID-19 Vaccine Gets FDA Approval, Use Significantly Curtailed. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Assessing The Backup Qbs Who Could Spearhead An Nfl Playoff Team In 2024
May 21, 2025
Assessing The Backup Qbs Who Could Spearhead An Nfl Playoff Team In 2024
May 21, 2025 -
 Maple Leafs Season Ends As Panthers Cruise To Game 7 Victory
May 21, 2025
Maple Leafs Season Ends As Panthers Cruise To Game 7 Victory
May 21, 2025 -
 Mma World Reacts Jon Jones Controversial Strip The Duck Comment On Tom Aspinall
May 21, 2025
Mma World Reacts Jon Jones Controversial Strip The Duck Comment On Tom Aspinall
May 21, 2025 -
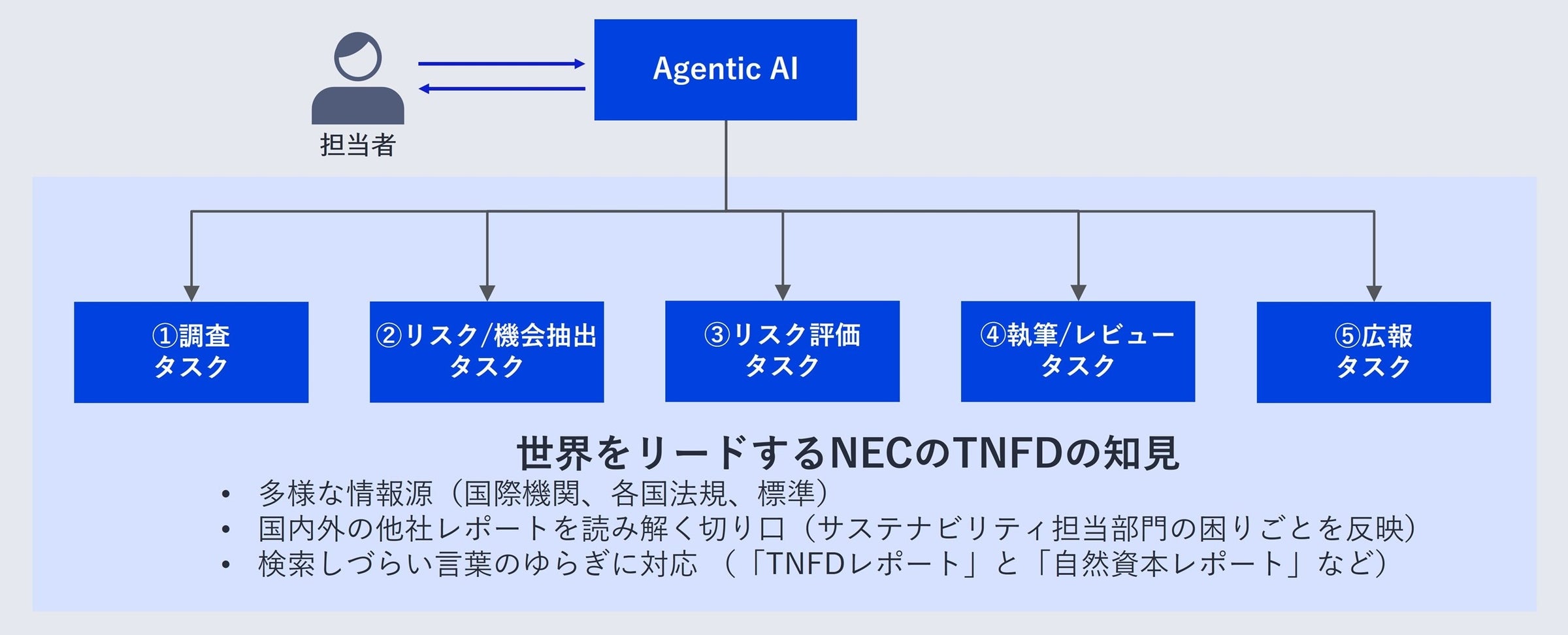 Agentic Ai Nec Ai Tnfd
May 21, 2025
Agentic Ai Nec Ai Tnfd
May 21, 2025 -
 Tyrese Haliburton Extends Olive Branch Invites Heckling Fan Back
May 21, 2025
Tyrese Haliburton Extends Olive Branch Invites Heckling Fan Back
May 21, 2025
