Novavax's COVID-19 Vaccine Gets FDA Nod, But With Key Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax's COVID-19 Vaccine Gets FDA Nod, but with Key Restrictions
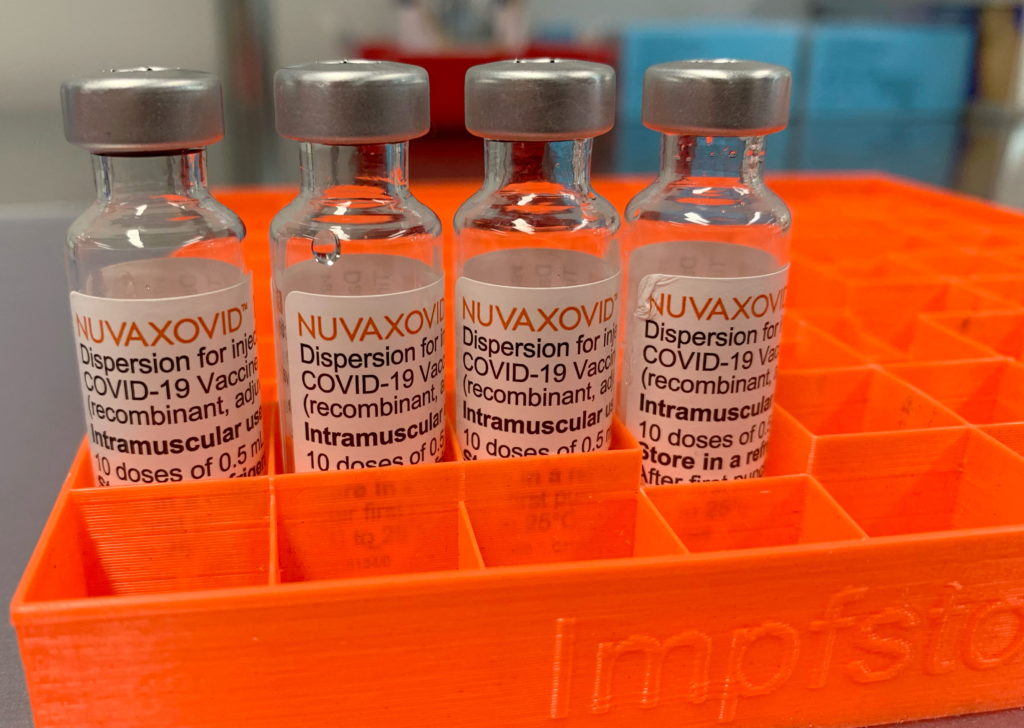
Novavax's COVID-19 vaccine, Nuvaxovid, has finally received Emergency Use Authorization (EUA) from the Food and Drug Administration (FDA), marking a significant development in the fight against the pandemic. However, the approval comes with crucial limitations, raising questions about its widespread adoption and future role in vaccination strategies.
The FDA's decision, announced [Insert Date of Announcement], grants authorization for the use of Nuvaxovid in individuals 18 years of age and older. This protein-subunit vaccine, a different technology from the mRNA vaccines like Pfizer-BioNTech and Moderna, has been eagerly awaited by some who expressed hesitancy towards the newer technologies. This approval offers an alternative option for those seeking a different type of COVID-19 vaccine.
Key Restrictions Limiting Nuvaxovid's Impact
Despite the approval, the FDA's EUA comes with several significant caveats that will likely limit the vaccine's immediate impact:
-
Limited Supply: The FDA authorization doesn't guarantee widespread availability. Novavax faces production challenges, meaning the vaccine may not be readily accessible to the broader population in the short term. This limited supply could hinder its contribution to ongoing vaccination efforts.
-
Efficacy Concerns: While the vaccine demonstrated efficacy in clinical trials, the data isn't as robust as that seen with the mRNA vaccines. The FDA's review acknowledges this, potentially raising concerns among healthcare providers and individuals considering vaccination. Further studies are needed to fully assess its long-term effectiveness and protection against emerging variants.
-
Target Audience: The EUA currently only covers adults 18 years and older. Approval for younger age groups remains pending, further restricting its potential reach and impact on achieving herd immunity.
What Does This Mean for the Future of COVID-19 Vaccination?
The approval of Nuvaxovid represents a step forward in diversifying the COVID-19 vaccine portfolio. The availability of a protein-subunit vaccine may help address vaccine hesitancy in certain populations who have been reluctant to receive mRNA vaccines. However, the limitations imposed by the FDA highlight the ongoing challenges in ensuring equitable access to effective COVID-19 vaccines globally.
The limited supply and less robust efficacy data compared to existing vaccines suggest that Nuvaxovid may not immediately become a cornerstone of vaccination campaigns. Its role may be more targeted, potentially benefiting specific populations or serving as a booster option once larger-scale data on its long-term effectiveness is available.
Looking Ahead: Next Steps for Novavax and Public Health Officials
Novavax will need to address the production challenges to ensure a sufficient supply of Nuvaxovid to meet potential demand. Further clinical trials are also crucial to solidify the vaccine's efficacy data and secure authorization for younger age groups.
Public health officials, in turn, must carefully consider the implications of these restrictions when integrating Nuvaxovid into existing vaccination strategies. Transparency about the vaccine's limitations will be key to maintaining public trust and ensuring informed decision-making.
The introduction of Nuvaxovid presents a complex scenario. While offering a choice, its restricted availability and data limitations mean its impact on the broader COVID-19 pandemic response remains to be seen. The coming months will be crucial in determining its long-term role in the global fight against COVID-19.
Keywords: Novavax, COVID-19 vaccine, Nuvaxovid, FDA approval, Emergency Use Authorization, EUA, vaccine hesitancy, protein-subunit vaccine, mRNA vaccine, vaccine efficacy, vaccine supply, COVID-19 pandemic, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax's COVID-19 Vaccine Gets FDA Nod, But With Key Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Exclusive Interview The Making Of Netflixs Documentary Fall Of Favre
May 20, 2025
Exclusive Interview The Making Of Netflixs Documentary Fall Of Favre
May 20, 2025 -
 Game 7 Clincher Panthers Sweep Aside Maple Leafs To Secure Playoff Win
May 20, 2025
Game 7 Clincher Panthers Sweep Aside Maple Leafs To Secure Playoff Win
May 20, 2025 -
 Lionel Messi Speaks Out Inter Miamis Struggles And The Path To Recovery
May 20, 2025
Lionel Messi Speaks Out Inter Miamis Struggles And The Path To Recovery
May 20, 2025 -
 Lost Pets Remembered Memorial Service After Funeral Home Cremains Incident
May 20, 2025
Lost Pets Remembered Memorial Service After Funeral Home Cremains Incident
May 20, 2025 -
 Weekend Wrecks Rock Indy 500 Practice
May 20, 2025
Weekend Wrecks Rock Indy 500 Practice
May 20, 2025
