Novavax's COVID-19 Vaccine: FDA Approval And Usage Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax's COVID-19 Vaccine: FDA Approval, Usage, and Limitations Explained
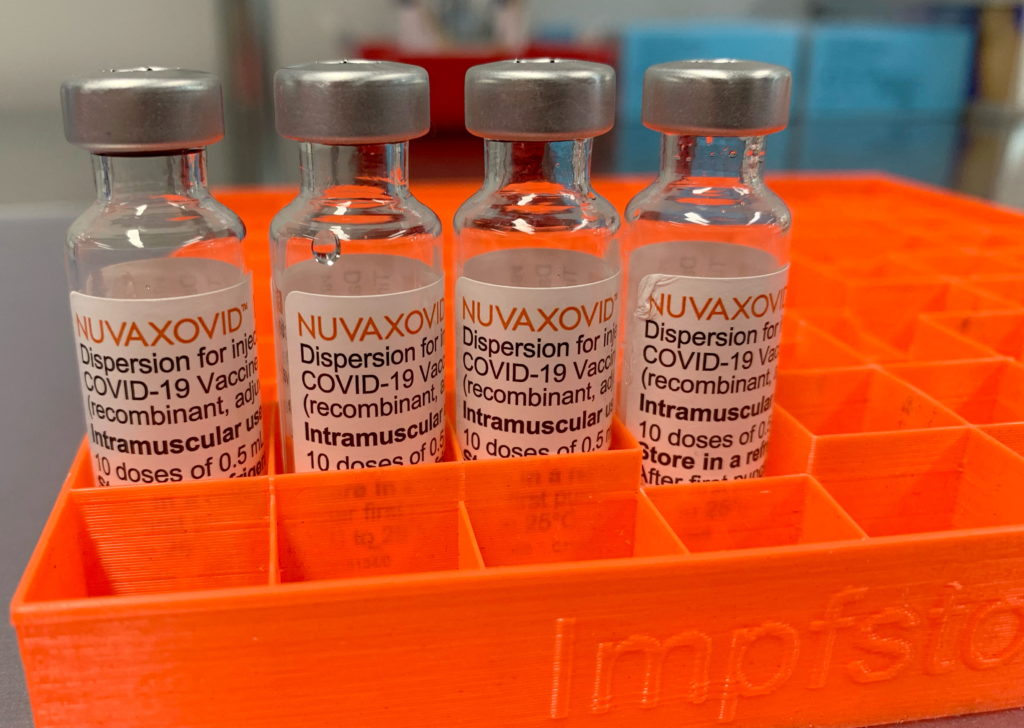
The arrival of Novavax's COVID-19 vaccine, Nuvaxovid (also known as Covovax internationally), marked a significant development in the global fight against the pandemic. Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, Novavax utilizes a more traditional protein subunit technology, sparking considerable interest among vaccine hesitant individuals. However, its FDA approval and subsequent usage have been accompanied by certain restrictions. This article will dissect the intricacies of Novavax's approval, its authorized use, and the limitations surrounding its deployment.
FDA Approval and Emergency Use Authorization (EUA): A Timeline
Novavax's journey to FDA approval was longer than its mRNA counterparts. While initially granted Emergency Use Authorization (EUA) in July 2022, full FDA approval arrived later. This delay stemmed from various factors, including rigorous clinical trial data analysis and manufacturing challenges. The full FDA approval solidified its place as a viable option within the COVID-19 vaccination landscape, albeit with specific caveats.
How Novavax Works: Understanding the Protein Subunit Technology
Unlike mRNA vaccines which instruct cells to produce viral proteins, Novavax's vaccine uses purified, lab-made pieces of the SARS-CoV-2 virus (the spike protein). This protein is then combined with an adjuvant – a substance that enhances the immune response. This traditional approach is familiar to many, potentially alleviating some concerns associated with newer vaccine technologies. This method makes it a potentially attractive alternative for individuals who may be hesitant about mRNA vaccines.
Authorized Use and Target Population
The FDA authorized Nuvaxovid for individuals aged 18 years and older. Initial recommendations focused on offering an alternative choice for those who might prefer a non-mRNA vaccine. However, evolving guidelines may see its use expanded or restricted based on emerging data and variant prevalence. It's crucial to consult with healthcare professionals for personalized recommendations.
Limitations and Considerations:
While Novavax offers a valuable addition to the vaccine arsenal, certain limitations need to be acknowledged:
- Efficacy Rates: While effective, its efficacy rates against different COVID-19 variants, particularly Omicron subvariants, may be slightly lower compared to some mRNA vaccines. Ongoing studies continue to monitor its effectiveness against emerging strains.
- Storage and Handling: Novavax requires specific storage and handling conditions, potentially presenting logistical challenges compared to some other vaccines. This may affect its accessibility in certain regions or healthcare settings.
- Adverse Effects: As with any vaccine, Novavax can cause side effects, although these are generally mild and transient. Common side effects include pain at the injection site, fatigue, headache, muscle aches, and joint pain. Severe allergic reactions are rare but possible.
Conclusion: A Valuable Addition to the COVID-19 Vaccine Strategy
Novavax's COVID-19 vaccine provides a valuable alternative for individuals seeking a non-mRNA option. However, understanding its FDA approval process, authorized usage, and limitations is paramount. Consulting with a healthcare provider to discuss individual risk factors and vaccine suitability remains crucial in making informed decisions regarding COVID-19 vaccination. Staying updated on the latest recommendations from the CDC and FDA is equally important to ensure you have the most current information.
Disclaimer: This article provides general information and should not be considered medical advice. Consult with your healthcare provider for personalized recommendations. This article is for informational purposes only and does not endorse any specific vaccine or manufacturer.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, Covovax, protein subunit vaccine, mRNA vaccine, vaccine efficacy, vaccine side effects, vaccine safety, COVID-19 vaccination, vaccine hesitancy, coronavirus vaccine.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax's COVID-19 Vaccine: FDA Approval And Usage Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Exclusive Interview The Making Of Netflixs Fall Of Favre And The Story Behind It
May 20, 2025
Exclusive Interview The Making Of Netflixs Fall Of Favre And The Story Behind It
May 20, 2025 -
 Galactuss Terrifying Stature A Look At The New Fantastic Four Promo Art
May 20, 2025
Galactuss Terrifying Stature A Look At The New Fantastic Four Promo Art
May 20, 2025 -
 North Texas Weather Forecast Post Storm Calm Before Tuesdays Cold Front
May 20, 2025
North Texas Weather Forecast Post Storm Calm Before Tuesdays Cold Front
May 20, 2025 -
 First Look At Franklin Richards Analysis Of New Fantastic Four Merchandise
May 20, 2025
First Look At Franklin Richards Analysis Of New Fantastic Four Merchandise
May 20, 2025 -
 Multiple Crashes Rock Indy 500 Preparations
May 20, 2025
Multiple Crashes Rock Indy 500 Preparations
May 20, 2025
