Novavax Vaccine Gets FDA Nod, Yet Faces Unusual Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax Vaccine Gets FDA Nod, Yet Faces Unusual Usage Restrictions
The FDA has finally granted full approval to the Novavax COVID-19 vaccine, a move many hailed as a victory for vaccine choice. However, the approval comes with a significant caveat: unusual and arguably restrictive usage guidelines that have left experts and the public questioning the decision. This development raises important questions about vaccine accessibility and the future of the COVID-19 vaccine landscape.
A Long-Awaited Approval, But With Strings Attached
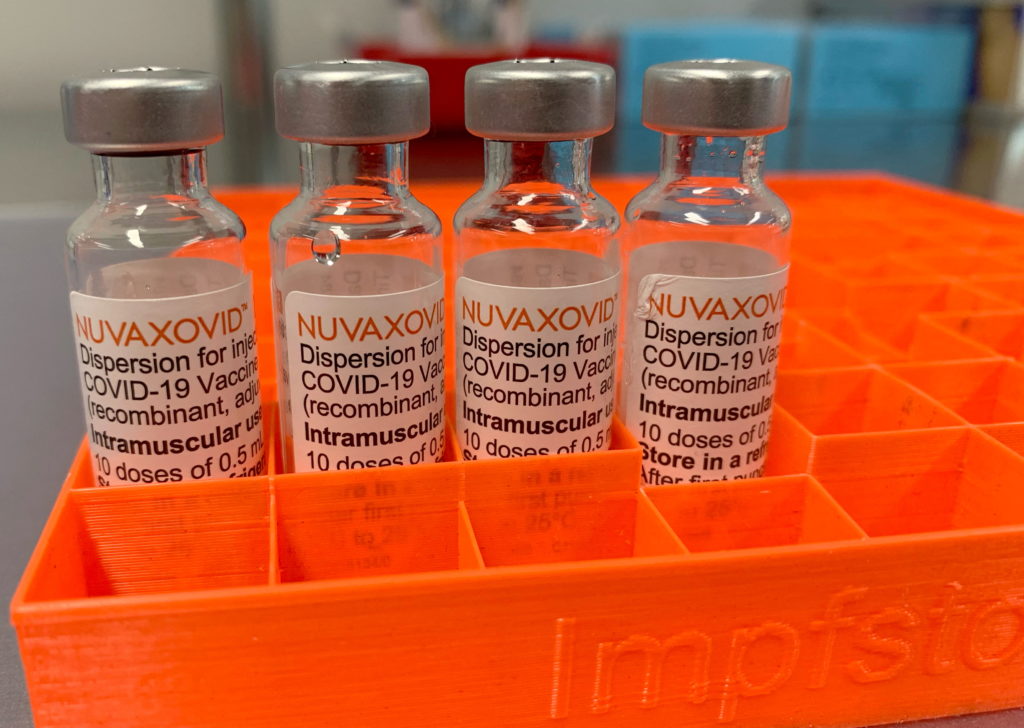
After months of delay and anticipation, the Novavax COVID-19 vaccine, marketed under the brand name Nuvaxovid, received full approval from the Food and Drug Administration (FDA) for individuals 18 years and older. This approval marks a significant milestone for Novavax, who initially faced production hurdles and regulatory challenges. The protein-subunit vaccine, a technology different from the mRNA vaccines produced by Pfizer-BioNTech and Moderna, was touted as an alternative for individuals hesitant about mRNA technology.
However, the FDA's approval isn't unconditional. The agency has imposed significant usage restrictions, limiting its recommended use to specific populations and scenarios. This contrasts sharply with the broader authorization granted to other COVID-19 vaccines. The precise reasons behind these limitations remain unclear, sparking debate among healthcare professionals and public health officials.
Understanding the Unusual Restrictions
While the FDA hasn't explicitly detailed all the reasons for the restricted usage, several factors are likely contributing:
- Limited Demand: With other COVID-19 vaccines already widely available and the pandemic's acute phase waning, demand for a new vaccine might be lower than anticipated. This could contribute to the FDA’s decision to focus its recommendation on specific demographics.
- Efficacy Data: While effective, the Novavax vaccine's efficacy data might not demonstrate a clear advantage over existing vaccines in all populations. Further research may be needed to fully understand its performance in various demographics.
- Supply Chain Considerations: Production and distribution of the Novavax vaccine might present logistical challenges, influencing the FDA’s decision on its targeted deployment.
Concerns and Future Implications
The restrictive usage guidelines raise concerns about vaccine equity and access. If the vaccine is only recommended for specific groups, it could limit its overall impact and potentially exacerbate existing health disparities. Furthermore, the limited availability may discourage wider adoption, even among those who prefer protein-subunit vaccines.
The FDA's decision highlights the complex interplay between scientific data, public health needs, and regulatory approvals. The long-term implications of these restrictions remain to be seen, but they underscore the ongoing evolution of the COVID-19 vaccine landscape and the need for continued transparency and communication.
What This Means for You
While the full FDA approval is positive news for Novavax, the accompanying restrictions mean this vaccine might not be readily available to everyone. Consult with your healthcare provider to determine which COVID-19 vaccine is most appropriate for your individual needs and circumstances. Staying informed about vaccine updates and guidelines from reliable sources like the and is crucial.
This situation underscores the importance of continued research and monitoring of all COVID-19 vaccines to ensure their safety and efficacy across diverse populations. The future of the Novavax vaccine and its role in global vaccination efforts will depend heavily on how these usage restrictions evolve.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax Vaccine Gets FDA Nod, Yet Faces Unusual Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Nuggets Adelman Receives Player Backing Amidst Rising Accolades
May 20, 2025
Nuggets Adelman Receives Player Backing Amidst Rising Accolades
May 20, 2025 -
 Jamie Lee Curtis Discusses Her Close Relationship With Lindsay Lohan
May 20, 2025
Jamie Lee Curtis Discusses Her Close Relationship With Lindsay Lohan
May 20, 2025 -
 Twins Collision Carlos Correa And Byron Buxton Suffer Concussions
May 20, 2025
Twins Collision Carlos Correa And Byron Buxton Suffer Concussions
May 20, 2025 -
 Better World Gala At Cannes Kevin Spaceys Return To The Spotlight
May 20, 2025
Better World Gala At Cannes Kevin Spaceys Return To The Spotlight
May 20, 2025 -
 Post Pectra Upgrade Ethereum Attracts 200 Million In Fresh Investment
May 20, 2025
Post Pectra Upgrade Ethereum Attracts 200 Million In Fresh Investment
May 20, 2025
