Novavax COVID-19 Vaccine Gets FDA Nod, Yet Faces Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
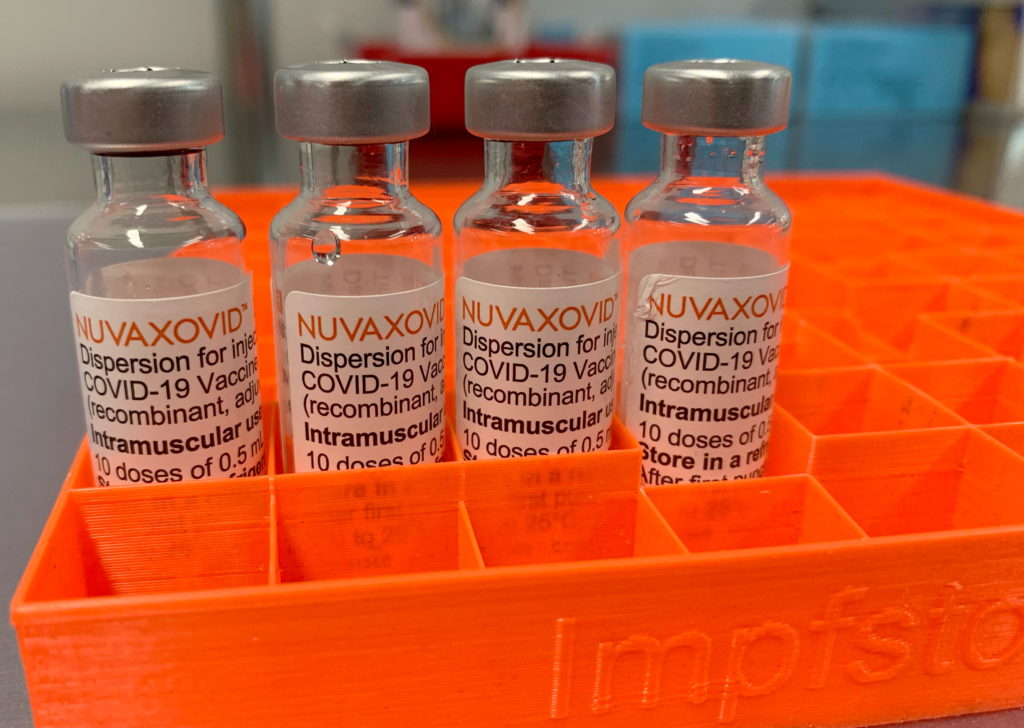
Novavax COVID-19 Vaccine Gets FDA Nod, But Faces Usage Restrictions
The FDA has finally granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, offering another option in the fight against the pandemic. However, its approval comes with significant usage restrictions, limiting its immediate impact. This development marks a significant milestone for Novavax, but also raises questions about the vaccine's place in the current vaccination landscape.
A Protein Subunit Vaccine: A Different Approach
Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, or the viral vector vaccines like Johnson & Johnson's Janssen, Nuvaxovid is a protein subunit vaccine. This technology uses purified fragments of the virus to trigger an immune response, potentially appealing to individuals hesitant about mRNA or viral vector technology. This "traditional" approach has been associated with potentially fewer side effects for some individuals, although more research is needed to confirm this across diverse populations.
FDA Approval, But With Caveats
The FDA's approval is not without limitations. The EUA is specifically for individuals aged 18 years and older, excluding children and adolescents. Furthermore, the vaccine is authorized only for use as a two-dose primary series, with no current authorization for booster shots. This contrasts with the readily available booster shots for other authorized COVID-19 vaccines. The limited age range and lack of booster authorization significantly restrict the vaccine's immediate applicability.
Why the Restrictions?
The FDA's cautious approach likely stems from several factors. Firstly, the clinical trial data supporting the vaccine's efficacy, while positive, might not be as extensive as that for other already-authorized vaccines. Secondly, the evolving COVID-19 landscape, with new variants constantly emerging, necessitates careful evaluation of the vaccine's effectiveness against these variants. Finally, the relatively late entry of Nuvaxovid into the market means its rollout will face competition from existing vaccines with established distribution networks and widespread public acceptance.
Market Implications and Future Prospects
The arrival of Nuvaxovid could still impact the global vaccination efforts, particularly in countries with limited access to mRNA vaccines or those with populations who prefer protein subunit vaccines. However, the restricted usage will likely limit its initial impact in developed countries where other vaccines are widely available and boosted immunity is already prevalent. The future success of Nuvaxovid hinges on further clinical trials to expand its authorized use to younger age groups and to demonstrate its efficacy against emerging variants, potentially paving the way for booster authorization. Novavax will also need to focus on efficient distribution and public awareness campaigns to ensure its uptake.
Looking Ahead: Uncertainties Remain
While the FDA's authorization is a positive step for Novavax, the substantial usage restrictions highlight the complexities of vaccine development and deployment. The long-term impact of Nuvaxovid remains uncertain, contingent on further research and the evolving dynamics of the COVID-19 pandemic. Only time will tell whether this new vaccine will significantly alter the existing vaccination landscape. Further updates from clinical trials and regulatory bodies will be crucial in determining the vaccine's ultimate role in global COVID-19 vaccination strategies.
Keywords: Novavax, COVID-19 vaccine, FDA approval, EUA, Nuvaxovid, protein subunit vaccine, vaccine restrictions, mRNA vaccine, COVID-19 vaccination, vaccine efficacy, vaccine rollout, global vaccination, pandemic
(Note: This article is for informational purposes only and does not constitute medical advice. Consult with a healthcare professional for any health concerns or before making any decisions related to your health or treatment.)

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Yet Faces Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Mark Scheifeles Grief And Grit Nhl Player Scores Amid Family Tragedy
May 20, 2025
Mark Scheifeles Grief And Grit Nhl Player Scores Amid Family Tragedy
May 20, 2025 -
 Nine Days Later Christen Press Leaves Hospital Following Angel City Game Collapse
May 20, 2025
Nine Days Later Christen Press Leaves Hospital Following Angel City Game Collapse
May 20, 2025 -
 Christen Presss Recovery Update Released From Hospital Following Angel City Game Incident
May 20, 2025
Christen Presss Recovery Update Released From Hospital Following Angel City Game Incident
May 20, 2025 -
 Moodys Downgrade Unfazed S And P 500 Extends Winning Streak Market Shows Resilience
May 20, 2025
Moodys Downgrade Unfazed S And P 500 Extends Winning Streak Market Shows Resilience
May 20, 2025 -
 Mlb Best Bets Today Walk Off Wagers For White Sox Vs Cubs And More
May 20, 2025
Mlb Best Bets Today Walk Off Wagers For White Sox Vs Cubs And More
May 20, 2025
