Novavax COVID-19 Vaccine Gets FDA Nod, Subject To Strict Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
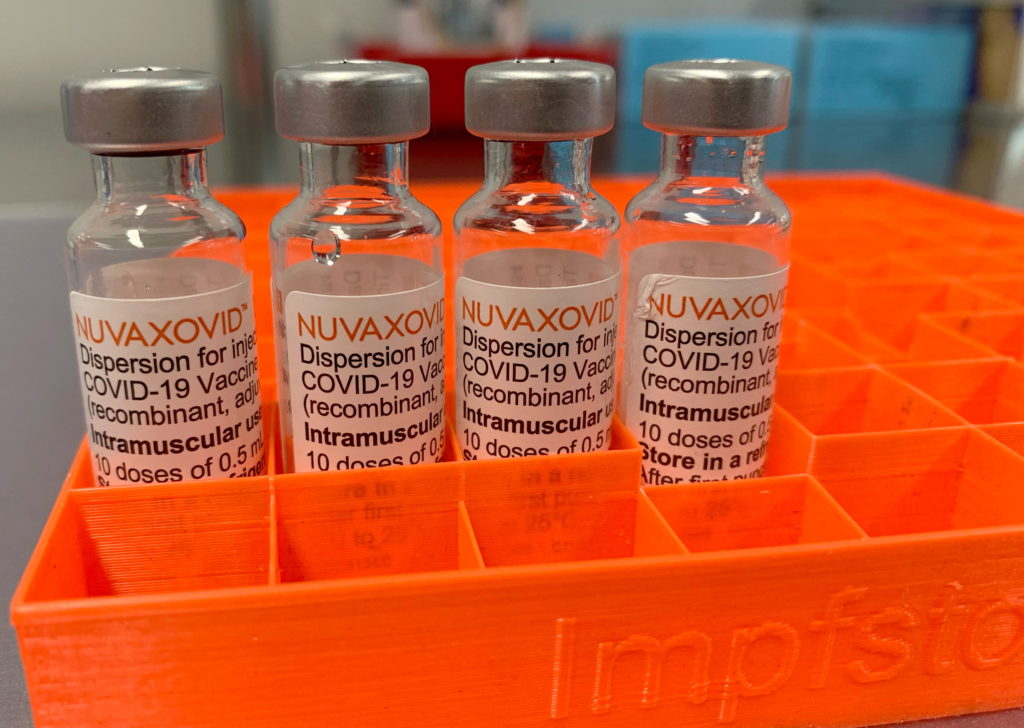
Novavax COVID-19 Vaccine Gets FDA Nod, But Under Strict Conditions
The FDA has finally granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, but with significant stipulations. This long-awaited approval offers a new option for individuals hesitant about mRNA vaccines, but its rollout will be carefully monitored. The decision marks a significant development in the fight against the pandemic, providing another tool in the arsenal against COVID-19, but the conditions attached highlight the agency's cautious approach.
What are the key conditions imposed by the FDA?
The FDA's authorization is not without strings attached. The agency is requiring robust post-market surveillance to carefully track the vaccine's safety and effectiveness. This includes stringent monitoring for rare adverse events, something particularly crucial given the relatively later entry of this vaccine into the market. This intense scrutiny is a direct response to the need for ongoing data collection, especially concerning potential long-term side effects. The FDA will be closely analyzing data from real-world use to ensure the vaccine continues to meet its safety and efficacy standards.
Why the cautious approach?
The FDA's cautious approach is understandable. While Novavax's protein-based vaccine technology has been used for decades in other vaccines, its late arrival in the COVID-19 vaccine landscape necessitates meticulous monitoring. The mRNA vaccines from Pfizer-BioNTech and Moderna have already amassed a vast amount of real-world data, providing a stronger safety profile based on extensive use. Novavax, therefore, faces a higher bar to demonstrate its safety and efficacy profile in a similar way.
What makes the Novavax vaccine different?
Unlike the mRNA vaccines, Novavax uses a more traditional protein-based technology. The vaccine uses a lab-made version of the spike protein found on the surface of the SARS-CoV-2 virus. This spike protein is then combined with an adjuvant to boost the immune response. This approach may appeal to individuals hesitant about mRNA vaccines, providing a different option for vaccination. Many people have expressed concerns about the novel mRNA technology; this offers a more familiar approach.
What does this mean for the public?
This EUA opens up vaccination opportunities for a segment of the population who may have been hesitant to receive an mRNA vaccine. However, the public should be aware that the FDA’s stringent conditions reflect a necessary level of caution. The vaccine's availability will likely be phased, allowing for close monitoring of its effects in the real world. This phased rollout allows for timely intervention should any safety concerns arise.
Looking Ahead:
The FDA's decision is a step forward in the global effort to combat COVID-19. However, the conditions attached highlight the importance of ongoing surveillance and the need for a nuanced approach to vaccine development and deployment. The long-term impact of this approval will depend on how effectively the post-market surveillance program is implemented and the vaccine's uptake within the population. For reliable updates and information about the Novavax vaccine, consult official sources like the and the . Staying informed is crucial in navigating the evolving COVID-19 landscape.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Subject To Strict Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 New Fantastic Four Merch Hints At Franklin Richards Role Questions About Sue Storms Son
May 20, 2025
New Fantastic Four Merch Hints At Franklin Richards Role Questions About Sue Storms Son
May 20, 2025 -
 Bali Calls For Global Cooperation To Enhance Tourist Safety And Respect
May 20, 2025
Bali Calls For Global Cooperation To Enhance Tourist Safety And Respect
May 20, 2025 -
 Urgent Peace Push Trump Initiates Russia Ukraine Truce Talks
May 20, 2025
Urgent Peace Push Trump Initiates Russia Ukraine Truce Talks
May 20, 2025 -
 Ufc Fans Erupt Over Jon Jones Controversial Strip The Duck Comment On Aspinall
May 20, 2025
Ufc Fans Erupt Over Jon Jones Controversial Strip The Duck Comment On Aspinall
May 20, 2025 -
 New Rules Target Bad Tourist Behavior In Bali What You Need To Know
May 20, 2025
New Rules Target Bad Tourist Behavior In Bali What You Need To Know
May 20, 2025
