Novavax COVID-19 Vaccine Gets FDA Nod, Specific Use Restrictions Apply
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Receives FDA Approval: Specific Use Restrictions Apply
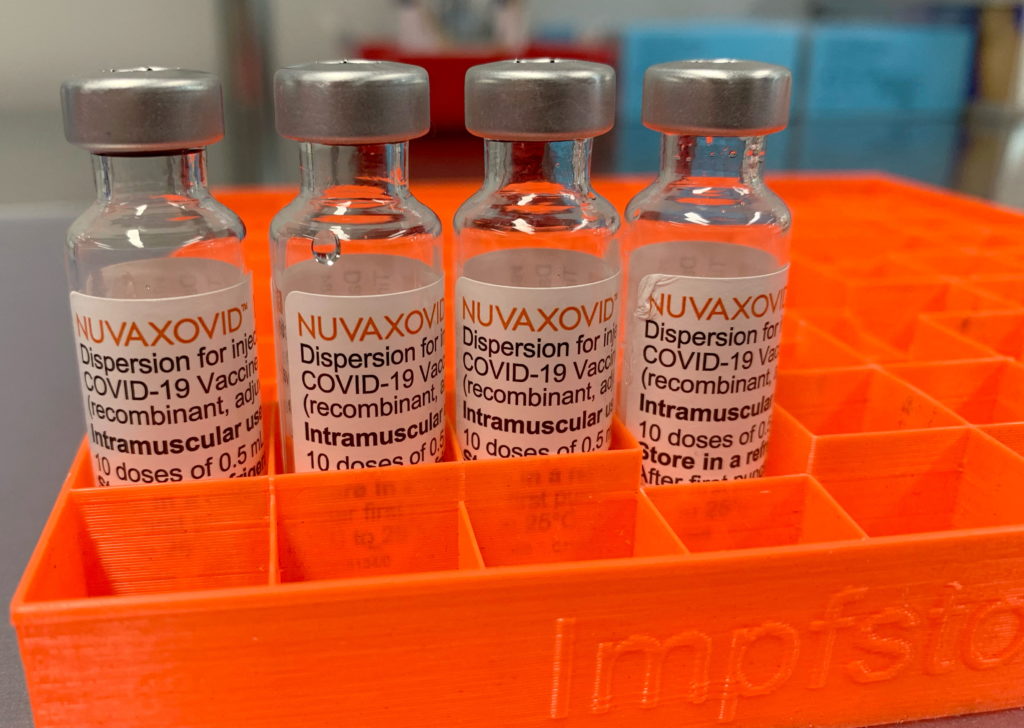
The FDA has granted full approval to the Novavax COVID-19 vaccine, Nuvaxovid, but with specific use restrictions. This landmark decision comes after months of review and offers a new option for individuals seeking COVID-19 vaccination. However, it's crucial to understand the limitations and who this vaccine is most suitable for.
The approval of Nuvaxovid, a protein-subunit vaccine, marks a significant step in the ongoing fight against the COVID-19 pandemic. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, Novavax uses a more traditional approach, employing a lab-made version of the virus's spike protein to trigger an immune response. This difference could be a key factor for individuals hesitant about mRNA technology.
Understanding the FDA Approval and its Limitations
The FDA's full approval is a significant achievement, signifying a rigorous review process confirming the vaccine's safety and effectiveness. However, the approval isn't blanket authorization. The FDA has placed specific use restrictions, primarily focusing on the vaccine's authorized use in individuals 18 years of age and older. This contrasts with the wider age range authorized for other COVID-19 vaccines currently on the market.
This age restriction is based on the available clinical trial data, which primarily focused on adults. Further studies are needed to assess the vaccine's efficacy and safety in younger populations. Therefore, parents should not expect this vaccine to be an option for their children at this time.
Why the Novavax Vaccine Matters
The introduction of the Novavax vaccine provides a valuable alternative for several reasons:
- Traditional Vaccine Technology: For individuals wary of mRNA technology, the protein-subunit approach may offer increased comfort and confidence.
- Potential for Broadening Vaccination Rates: The availability of a different vaccine type may encourage vaccine hesitancy among those who have previously resisted vaccination.
- Addressing Vaccine Supply Chain: Adding another vaccine to the market could potentially alleviate supply chain pressures and increase access to vaccination globally.
Who Should Consider the Novavax Vaccine?
While the Novavax vaccine offers a new option, it's not necessarily the right choice for everyone. Individuals should consult their healthcare provider to discuss the best vaccine option based on their individual health history, risk factors, and preferences. The decision should be a collaborative one between the patient and their doctor.
This vaccine might be a particularly good option for:
- Individuals with concerns about mRNA vaccines: The different technology used in the Novavax vaccine may address concerns some have about mRNA vaccines.
- Adults who have previously been hesitant to receive a COVID-19 vaccine: This new option provides another choice.
Staying Informed About COVID-19 Vaccination
The COVID-19 pandemic continues to evolve. Staying updated on the latest recommendations from health authorities like the and the is crucial. These organizations provide valuable information on vaccine safety, efficacy, and the latest guidance on COVID-19 prevention and treatment.
Remember to consult your healthcare provider for personalized advice on COVID-19 vaccination. They can help you understand the risks and benefits of different vaccines and determine the best course of action for your individual needs. Don't hesitate to ask questions and discuss your concerns. Your health is paramount.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Specific Use Restrictions Apply. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Schefflers Driver Scandal Latest Updates Before The Pga Championship
May 20, 2025
Schefflers Driver Scandal Latest Updates Before The Pga Championship
May 20, 2025 -
 Update Christen Press Discharged Following Angel City Soccer Match Collapse
May 20, 2025
Update Christen Press Discharged Following Angel City Soccer Match Collapse
May 20, 2025 -
 Can These Backup Quarterbacks Lead An Nfl Team To A Playoff Victory In 2024
May 20, 2025
Can These Backup Quarterbacks Lead An Nfl Team To A Playoff Victory In 2024
May 20, 2025 -
 Institutional Investors Drive 5 Billion Bitcoin Etf Boom
May 20, 2025
Institutional Investors Drive 5 Billion Bitcoin Etf Boom
May 20, 2025 -
 The Unexpected Friendship Jamie Lee Curtis And Lindsay Lohan
May 20, 2025
The Unexpected Friendship Jamie Lee Curtis And Lindsay Lohan
May 20, 2025
