Novavax COVID-19 Vaccine Gets FDA Nod, But With Unconventional Usage Limits
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, but with Unconventional Usage Limits
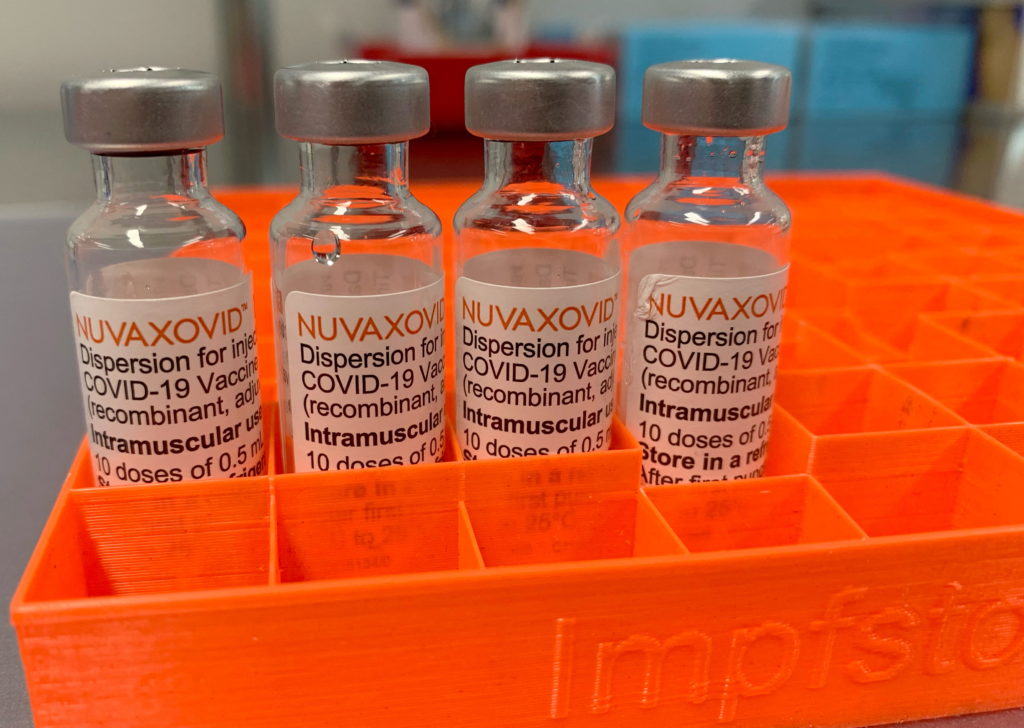
The FDA has finally granted full approval to the Novavax COVID-19 vaccine, Nuvaxovid, but with a significant caveat: its use is limited to individuals 18 years and older who are not eligible for or have declined other authorized or approved COVID-19 vaccines. This unconventional restriction raises questions about the vaccine's market impact and its role in the ongoing pandemic response.
The approval, announced [Insert Date], marks a significant milestone for Novavax, ending a long regulatory journey. However, the FDA's decision to significantly limit its usage is a surprising twist. Unlike other widely used vaccines like Pfizer-BioNTech and Moderna, Nuvaxovid won't be a first-line option for most Americans.
Why the Restricted Approval?
The FDA's decision stems from several factors. While Nuvaxovid demonstrated efficacy and safety in clinical trials, its arrival on the market is considerably later than other COVID-19 vaccines. By the time the FDA completed its review, the landscape of COVID-19 vaccination had already shifted significantly. The widespread availability of mRNA vaccines, along with high vaccination rates in many populations, reduced the urgency for a new vaccine with a restricted target audience.
The FDA's press release emphasized that Nuvaxovid's approval is based on its demonstrated safety and efficacy profile, but that its limited use reflects the current state of the COVID-19 pandemic and the availability of other vaccines. The agency also noted that [mention any specific data points regarding efficacy and side effects mentioned in the FDA release].
What Does This Mean for Novavax?
This limited approval presents a significant challenge for Novavax. While the full FDA approval lends credibility and might boost confidence in some individuals, the restricted usage severely limits its potential market reach. The company will likely need to focus its marketing efforts on a specific, albeit smaller, population segment. This could include individuals with specific concerns about mRNA vaccines or those who prefer a protein-based alternative.
The Future of Nuvaxovid
The long-term prospects for Nuvaxovid remain uncertain. The FDA’s decision highlights the dynamic nature of vaccine development and deployment during a pandemic. While the vaccine itself is safe and effective, its arrival late in the game means it faces a challenging market environment. The company may need to explore alternative strategies, such as focusing on international markets or adapting the vaccine for future variants. Further research on broader applications, perhaps as a booster for specific populations, could also play a role.
Further Reading:
- [Link to FDA Press Release]
- [Link to Novavax Website]
- [Link to CDC's COVID-19 Vaccine Information]
Conclusion:
The FDA's approval of the Novavax COVID-19 vaccine is a mixed bag. While the approval is a win for the company, the severely restricted usage limits its immediate impact. The decision underscores the constantly evolving landscape of pandemic response and the complexities involved in vaccine deployment. The future of Nuvaxovid hinges on the company's ability to adapt to this challenging situation and effectively target its limited market. Time will tell whether this vaccine finds a substantial niche within the COVID-19 vaccination strategy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Unconventional Usage Limits. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Assassins Creed Shadows Of London Ubisoft Addresses Player Concerns About Animal Killing
May 21, 2025
Assassins Creed Shadows Of London Ubisoft Addresses Player Concerns About Animal Killing
May 21, 2025 -
 Expect Showers And Chilly Temperatures All Week Long
May 21, 2025
Expect Showers And Chilly Temperatures All Week Long
May 21, 2025 -
 Ny Ag James Trump Under Fire Doj Investigation Criticized
May 21, 2025
Ny Ag James Trump Under Fire Doj Investigation Criticized
May 21, 2025 -
 Double Edged Sword Letitia James Navigates Trump And Doj Investigations
May 21, 2025
Double Edged Sword Letitia James Navigates Trump And Doj Investigations
May 21, 2025 -
 Indy 500 Betting Shwartzmans Pole A Game Changer For 2025 Odds
May 21, 2025
Indy 500 Betting Shwartzmans Pole A Game Changer For 2025 Odds
May 21, 2025
