Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
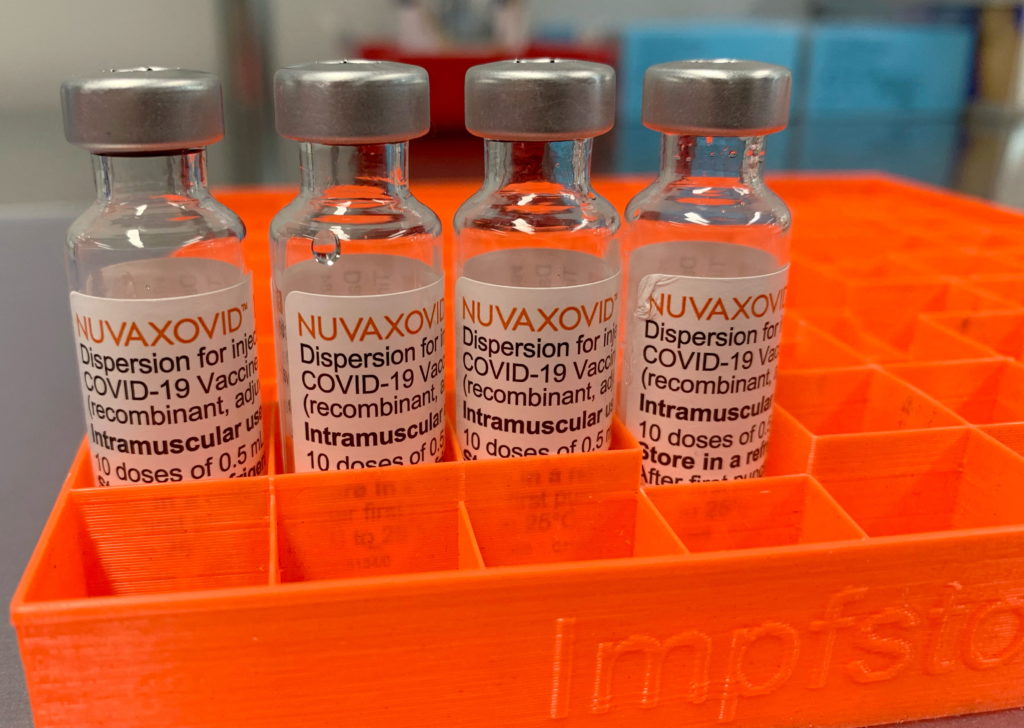
Novavax COVID-19 Vaccine Gets FDA Nod, but with Significant Usage Restrictions
The FDA has finally approved the Novavax COVID-19 vaccine, Nuvaxovid, but its rollout faces hurdles due to limited demand and significant usage restrictions. This approval marks a significant milestone, offering an alternative vaccine option for those hesitant about mRNA technology, but the conditional authorization comes with caveats that may limit its widespread adoption.
The long-awaited approval of Nuvaxovid, a protein-subunit vaccine, provides a different approach to COVID-19 immunization compared to the widely used mRNA vaccines from Pfizer-BioNTech and Moderna. This protein-based approach may appeal to individuals who have concerns about mRNA technology, potentially broadening vaccination rates. However, the FDA's decision comes with significant limitations, raising questions about the vaccine's practical impact on the ongoing pandemic.
Limited Demand and the Challenge of Market Entry
Despite the approval, the landscape for Nuvaxovid is challenging. The demand for COVID-19 vaccines has significantly decreased since the initial surge, leaving a smaller market for a new entrant. Furthermore, the high efficacy of existing vaccines and the widespread availability of booster shots have made the need for an additional vaccine less pressing for many. Novavax will face a tough battle to capture market share in a now-saturated market. This is further complicated by the vaccine's own limitations, discussed below.
Significant Usage Restrictions: Who Can and Cannot Get the Novavax Vaccine?
The FDA's conditional approval comes with several usage restrictions. While the vaccine is authorized for individuals 18 years and older, its use is largely restricted to those who:
-
Cannot receive mRNA vaccines: This is the primary target group for Novavax. Individuals with severe allergies to mRNA components or a history of adverse reactions might find Nuvaxovid a safer alternative. However, the FDA emphasizes this should only be considered after consultation with a healthcare provider.
-
Prefer a protein-based vaccine: Some individuals may simply prefer a protein-subunit vaccine based on their personal preferences or beliefs. This is a less medically-driven reason, but nonetheless valid.
It's crucial to emphasize that the Novavax vaccine is not intended as a first-line choice for most individuals. The already high vaccination rates achieved with mRNA vaccines, coupled with their proven efficacy and widespread availability, make them the more readily accessible and recommended option in most cases.
The Future of Nuvaxovid: A Niche Player?
The FDA's approval of the Novavax COVID-19 vaccine is a noteworthy development in the fight against the pandemic, but its future remains uncertain. The significant usage restrictions and the reduced demand for COVID-19 vaccines suggest that Nuvaxovid may occupy a niche role, primarily serving individuals who cannot or choose not to receive mRNA vaccines.
While the availability of a different vaccine technology is undoubtedly beneficial, the practical impact of Nuvaxovid’s approval on the broader public health landscape is likely to be limited. This highlights the ever-evolving nature of vaccine development and the dynamic challenges of managing public health crises. Further studies and monitoring of the vaccine's real-world effectiveness will be crucial in assessing its long-term contribution to COVID-19 prevention.
Call to Action: Consult with your healthcare provider to discuss which COVID-19 vaccine is most appropriate for your individual circumstances. Staying informed about vaccine updates is crucial for making informed decisions regarding your health. For reliable information, refer to the and websites.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Santa Rosa Police Two Teenagers Face Charges After Church Defecation And Urination Incident
May 21, 2025
Santa Rosa Police Two Teenagers Face Charges After Church Defecation And Urination Incident
May 21, 2025 -
 Two Youths Face Charges After Church Break In And Unsanitary Act
May 21, 2025
Two Youths Face Charges After Church Break In And Unsanitary Act
May 21, 2025 -
 Nhl Referee Rooney Targets Playoff Return Following Ban
May 21, 2025
Nhl Referee Rooney Targets Playoff Return Following Ban
May 21, 2025 -
 Boston Celtics Offseason Moves Could Make Or Break Championship Bid
May 21, 2025
Boston Celtics Offseason Moves Could Make Or Break Championship Bid
May 21, 2025 -
 Inter Miamis Messi Speaks Hope And Determination Amidst Difficult Times
May 21, 2025
Inter Miamis Messi Speaks Hope And Determination Amidst Difficult Times
May 21, 2025
