Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
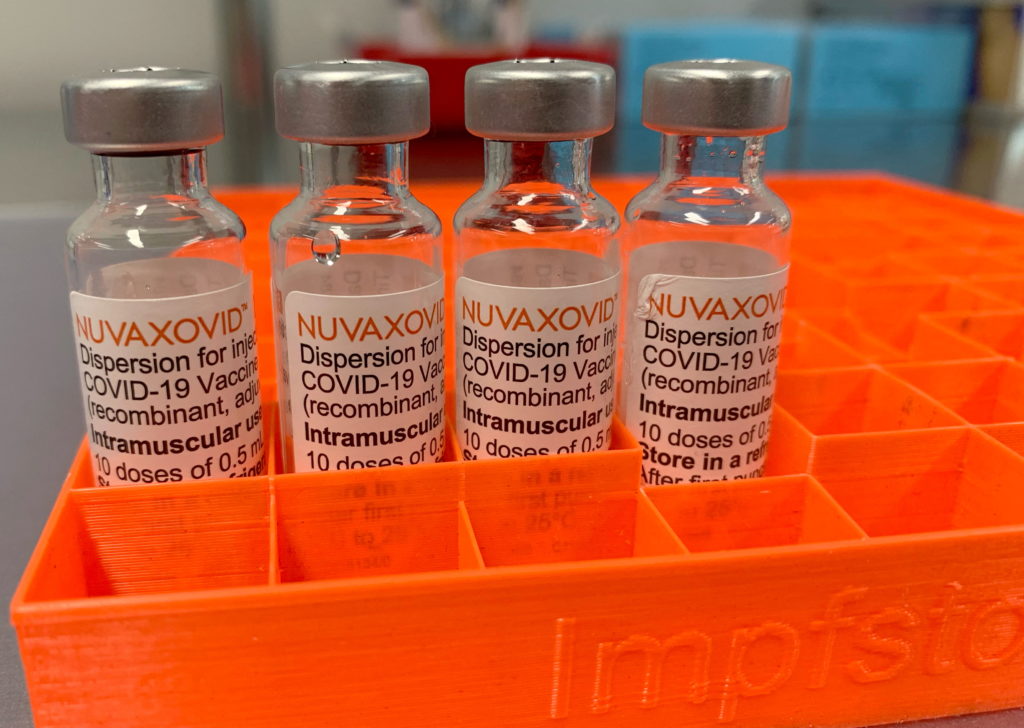
Novavax COVID-19 Vaccine Gets FDA Nod, but with Significant Usage Constraints
The FDA has finally granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, but its entry into the US market comes with significant caveats. While hailed as a victory for vaccine diversity, the limited circumstances under which it's authorized raise questions about its real-world impact.
A New Contender Enters the Ring, but with Restrictions
After months of anticipation and delays, the Novavax vaccine has received the green light from the FDA. This protein-subunit vaccine, a technology different from the mRNA vaccines developed by Pfizer-BioNTech and Moderna, offers a potentially valuable alternative for individuals hesitant about mRNA technology. This authorization provides an additional option for people seeking COVID-19 protection, but its rollout will be far from a widespread campaign.
Limited Authorization: Who Can Get the Novavax Vaccine?
The FDA's EUA is specifically for adults aged 18 years and older. This immediately limits its potential reach, excluding adolescents and children, a significant portion of the population. Furthermore, the authorization is not for primary vaccination series alone. It will be largely used for:
- Individuals who have not yet received a COVID-19 vaccine: While a welcome option for vaccine hesitant individuals, it's unclear how significant this population segment is now.
- Booster doses for individuals who have completed their primary vaccination series with other authorized or approved COVID-19 vaccines: This is a crucial aspect of the authorization, potentially widening the vaccine's utility. However, the effectiveness as a booster compared to existing boosters remains to be seen.
Why the Limited Scope?
Several factors contribute to the restricted authorization. Firstly, the data submitted by Novavax to the FDA showed lower efficacy rates compared to the mRNA vaccines, particularly against newer variants. While still effective in preventing severe disease, hospitalization, and death, the lower efficacy rates likely played a role in the cautious approach taken by the agency.
Secondly, the timing of the EUA is crucial. With COVID-19 cases declining and widespread vaccination already achieved using other vaccines, the demand for a new vaccine may be limited, further impacting its immediate impact.
What Does This Mean for the Future of COVID-19 Vaccination?
The Novavax vaccine's authorization marks a significant milestone in vaccine development. Its protein-subunit technology offers a different approach to vaccination, potentially appealing to individuals who were hesitant about mRNA vaccines. However, the limited scope of its EUA highlights the challenges of bringing a new vaccine to market in a rapidly evolving pandemic landscape.
The long-term impact of the Novavax vaccine remains uncertain. Its effectiveness against emerging variants will require ongoing monitoring and potential adjustments. Further studies are crucial to determining its efficacy in younger populations. While it offers a valuable alternative for some, its immediate impact on the broader COVID-19 vaccination effort might be less substantial than initially hoped.
Further Reading: For more information on COVID-19 vaccines, visit the .
Disclaimer: This article provides general information and should not be considered medical advice. Consult your healthcare provider for personalized guidance on COVID-19 vaccination.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Over 5 Billion Invested In Bitcoin Etfs Analyzing The Market Trend
May 20, 2025
Over 5 Billion Invested In Bitcoin Etfs Analyzing The Market Trend
May 20, 2025 -
 Curbing Misbehavior Bali Implements New Guidelines For Responsible Tourism
May 20, 2025
Curbing Misbehavior Bali Implements New Guidelines For Responsible Tourism
May 20, 2025 -
 Pga Championship Jon Rahms Positive Outlook After Crushing Finish
May 20, 2025
Pga Championship Jon Rahms Positive Outlook After Crushing Finish
May 20, 2025 -
 Helldivers 2 Warbond Event Masters Of Ceremony Drops Begin May 15th
May 20, 2025
Helldivers 2 Warbond Event Masters Of Ceremony Drops Begin May 15th
May 20, 2025 -
 Angel City Fcs Christen Press Released From Hospital Following On Field Collapse
May 20, 2025
Angel City Fcs Christen Press Released From Hospital Following On Field Collapse
May 20, 2025
