Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Caveats
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
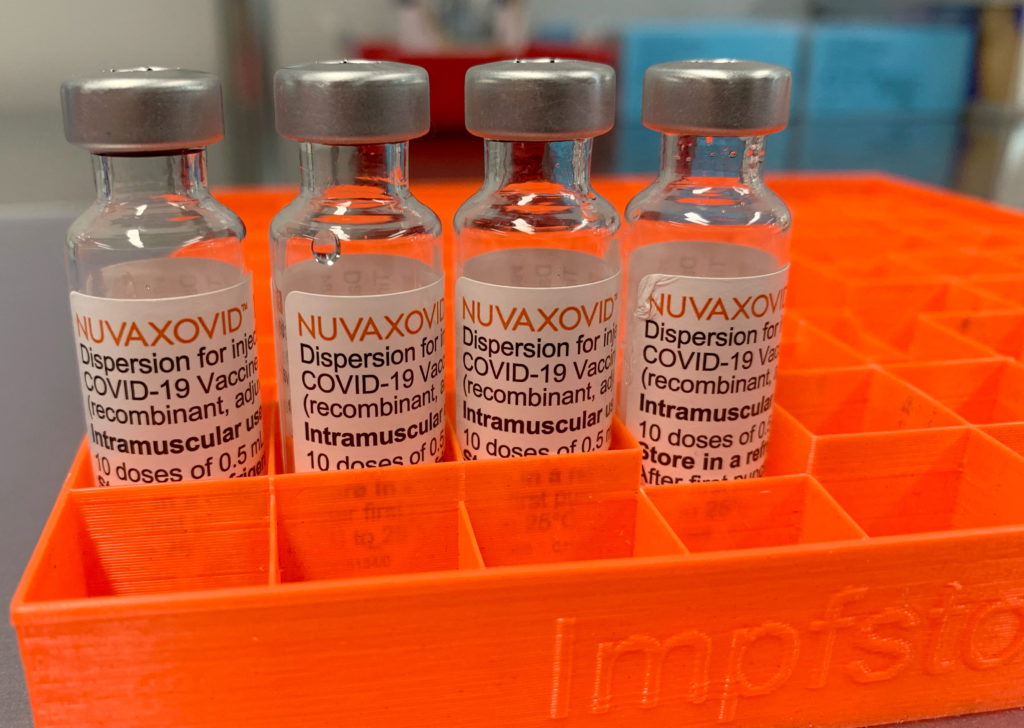
Novavax COVID-19 Vaccine Gets FDA Nod, but with Significant Caveats
The FDA has finally granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, but the announcement is tempered by significant caveats that limit its immediate impact. While this marks a victory for Novavax and offers another vaccine option, the authorization comes much later than other vaccines and with limitations that raise questions about its widespread adoption.
The delayed authorization raises concerns about the vaccine's potential market share, especially considering the widespread availability and high uptake of mRNA vaccines like Pfizer-BioNTech and Moderna. The limited use case specified by the FDA further complicates its rollout.
What are the FDA's Caveats?
The FDA's EUA comes with several key restrictions:
-
Limited Target Population: Initially, the authorization is primarily for adults 18 years of age and older. Expansion to younger age groups will depend on further clinical trial data. This contrasts sharply with the broader age ranges covered by other authorized COVID-19 vaccines.
-
Conditional Authorization: The EUA is conditional upon Novavax meeting specific post-authorization requirements, including ongoing safety monitoring and submission of additional data. This implies a degree of uncertainty regarding the vaccine's long-term safety and efficacy.
-
Lower Efficacy Compared to mRNA Vaccines: While Nuvaxovid showed efficacy against COVID-19 in clinical trials, the reported efficacy rates are lower than those observed with the mRNA vaccines. This difference needs further investigation and context within the evolving COVID-19 landscape. More detailed information regarding specific efficacy against various variants is eagerly awaited by public health officials.
-
Manufacturing and Distribution Challenges: The relatively late entry into the market could present challenges related to manufacturing capacity and distribution logistics. The global demand for COVID-19 vaccines has fluctuated, and it remains to be seen how much of a role Nuvaxovid will ultimately play.
Why is this a significant development, despite the caveats?
Despite these caveats, the FDA's approval is still a notable development for several reasons:
-
Vaccine Diversity: Offering another vaccine type diversifies the options available, potentially catering to individuals hesitant about mRNA vaccines due to concerns about their novel technology. This "vaccine hesitancy" remains a significant factor influencing vaccination rates globally.
-
Potential for Global Impact: Nuvaxovid's protein-subunit technology is a more traditional approach, making it potentially easier to manufacture and distribute in resource-limited settings. This could be crucial for improving global vaccination efforts, especially in low- and middle-income countries.
-
Future Variants: While the current efficacy data might be less impressive than mRNA vaccines, it is possible Nuvaxovid could prove more resilient against future COVID-19 variants. Further research is crucial to explore this possibility.
Looking Ahead: What's Next for Nuvaxovid?
The coming months will be critical in determining the true impact of Nuvaxovid. Novavax will need to address the FDA's concerns, conduct further research, and effectively navigate the challenges of manufacturing and distribution. The public health response to this new vaccine will also significantly influence its adoption and efficacy in combating the ongoing pandemic. Further analysis of its long-term efficacy and safety profile will be crucial to determining its place within the broader COVID-19 vaccination strategy. The global health community will be watching closely.
Call to Action: Stay informed about the latest developments regarding COVID-19 vaccines by consulting reputable sources like the CDC and WHO websites. Consult your healthcare provider to determine the most appropriate vaccination strategy for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Caveats. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Post Game Rivalry Trae Youngs Comments On Knicks And Thunder Fans
May 20, 2025
Post Game Rivalry Trae Youngs Comments On Knicks And Thunder Fans
May 20, 2025 -
 Robert Shwartzmans Road To The Indy 500 Pole Overcoming The Odds
May 20, 2025
Robert Shwartzmans Road To The Indy 500 Pole Overcoming The Odds
May 20, 2025 -
 Thunders Triumph Overcoming The Nuggets To Reach The West Finals
May 20, 2025
Thunders Triumph Overcoming The Nuggets To Reach The West Finals
May 20, 2025 -
 Maple Leafs Playoff Run Ends Panthers Triumph In Game 7
May 20, 2025
Maple Leafs Playoff Run Ends Panthers Triumph In Game 7
May 20, 2025 -
 Halls Winning Try Strategy Skill And A Touch Of Luck
May 20, 2025
Halls Winning Try Strategy Skill And A Touch Of Luck
May 20, 2025
