Novavax COVID-19 Vaccine Gets FDA Nod, But With Key Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
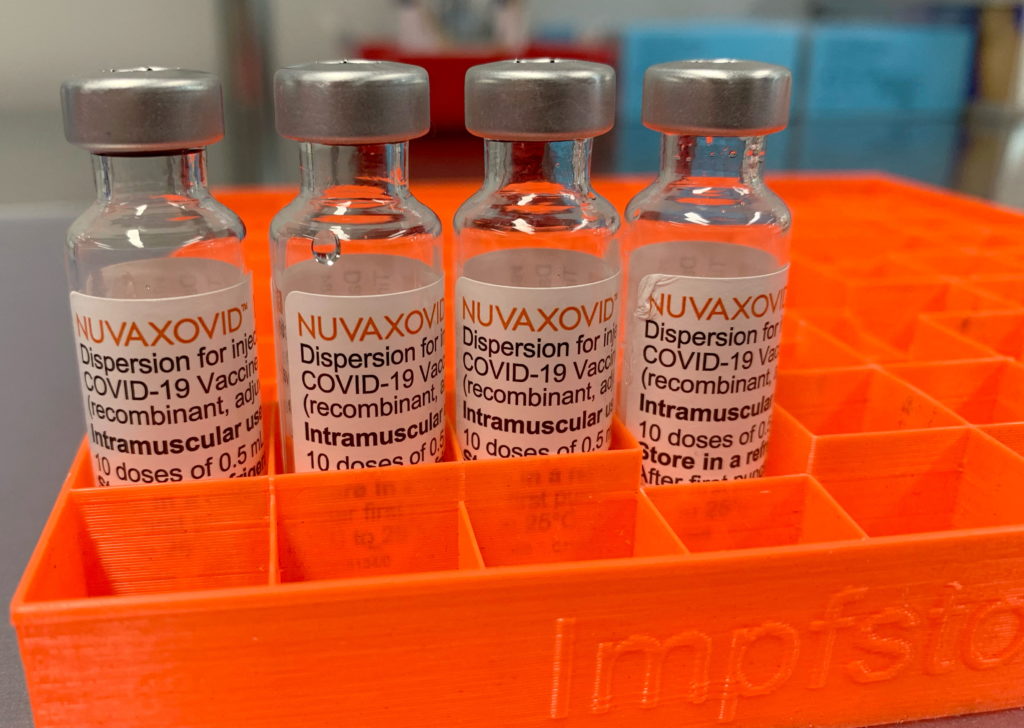
Novavax COVID-19 Vaccine Gets FDA Nod, but with Key Usage Constraints
The FDA has finally granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, marking a significant development in the fight against the pandemic. However, the authorization comes with crucial limitations, impacting its potential role in the ongoing vaccination efforts. This development raises questions about the vaccine's future market share and its place in a landscape already dominated by mRNA vaccines.
A Long-Awaited Approval, but with Caveats:
The approval, announced on July 13, 2023, follows a lengthy review process. Novavax, a protein subunit vaccine, represents a different technology compared to the widely used Pfizer-BioNTech and Moderna mRNA vaccines. This difference, while offering a potential alternative for individuals hesitant about mRNA technology, also contributes to the limited scope of the EUA.
The FDA's authorization is specifically for individuals 18 years of age and older. This differs from the mRNA vaccines, which have received authorization for use in younger age groups. Furthermore, the EUA is contingent on ongoing safety monitoring and the submission of further data by Novavax. This suggests a more cautious approach compared to the rapid authorization of the mRNA vaccines early in the pandemic.
Why the Limited Usage?
Several factors contribute to the constrained usage of the Novavax vaccine:
- Lower Efficacy Compared to mRNA Vaccines: While effective, clinical trials showed Nuvaxovid's efficacy to be slightly lower than that of the mRNA vaccines, particularly against newer variants. This difference likely influenced the FDA's decision to restrict its use.
- Late Entry into the Market: The vaccine's late arrival on the scene means it faces a saturated market. Many individuals have already received either two or more doses of mRNA vaccines, reducing the immediate demand for an alternative.
- Manufacturing Challenges: Novavax faced production delays during its development, potentially impacting its ability to meet the immediate demand even if the EUA had been granted sooner.
What Does This Mean for the Future of COVID-19 Vaccination?
The Novavax vaccine's approval provides a valuable option for individuals who prefer a protein-based vaccine over mRNA technology. For those with specific concerns or allergies related to mRNA vaccines, this offers an alternative route to COVID-19 protection. However, the limited EUA suggests that Nuvaxovid might not become a dominant player in the vaccination landscape.
The long-term impact will depend on several factors, including:
- Public acceptance: Will individuals actively seek out the Novavax vaccine given its constrained use and slightly lower efficacy compared to mRNA vaccines?
- Continued monitoring: The ongoing safety monitoring will be crucial in determining the long-term safety profile and efficacy of Nuvaxovid.
- Emerging variants: The vaccine's effectiveness against future variants will heavily influence its future role.
Conclusion:
The FDA's approval of the Novavax COVID-19 vaccine is a positive step, offering a choice for those who prefer a non-mRNA option. However, the limitations placed on its usage highlight the complexities of vaccine development and deployment. Time will tell whether this vaccine finds a significant niche in the ongoing battle against COVID-19. Further research and real-world data will be essential in assessing its true impact. For more information on COVID-19 vaccination, consult your healthcare provider or visit the CDC website: [Link to CDC website].

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Key Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Scottie Schefflers Dominant Performance At 2025 Pga Championship
May 20, 2025
Scottie Schefflers Dominant Performance At 2025 Pga Championship
May 20, 2025 -
 Ohtanis Strong Bullpen Session Follows Kershaws Rough Start
May 20, 2025
Ohtanis Strong Bullpen Session Follows Kershaws Rough Start
May 20, 2025 -
 Peaky Blinders Creator Reveals Plans For New Series With Key Departure
May 20, 2025
Peaky Blinders Creator Reveals Plans For New Series With Key Departure
May 20, 2025 -
 New Rules For Bali Tourists Addressing Misconduct And Protecting The Island
May 20, 2025
New Rules For Bali Tourists Addressing Misconduct And Protecting The Island
May 20, 2025 -
 Curbing Bad Tourist Behavior In Bali New Regulations And Their Impact
May 20, 2025
Curbing Bad Tourist Behavior In Bali New Regulations And Their Impact
May 20, 2025
