Novavax COVID-19 Vaccine Gets FDA Approval: Understanding The Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Approval: Understanding the Restrictions
The FDA has finally granted full approval to Novavax's COVID-19 vaccine, Nuvaxovid, marking a significant milestone in the fight against the pandemic. However, this approval comes with important restrictions, and understanding them is crucial for both healthcare providers and the public. This article delves into the details of the approval, highlighting the limitations and explaining what this means for the future of COVID-19 vaccination.
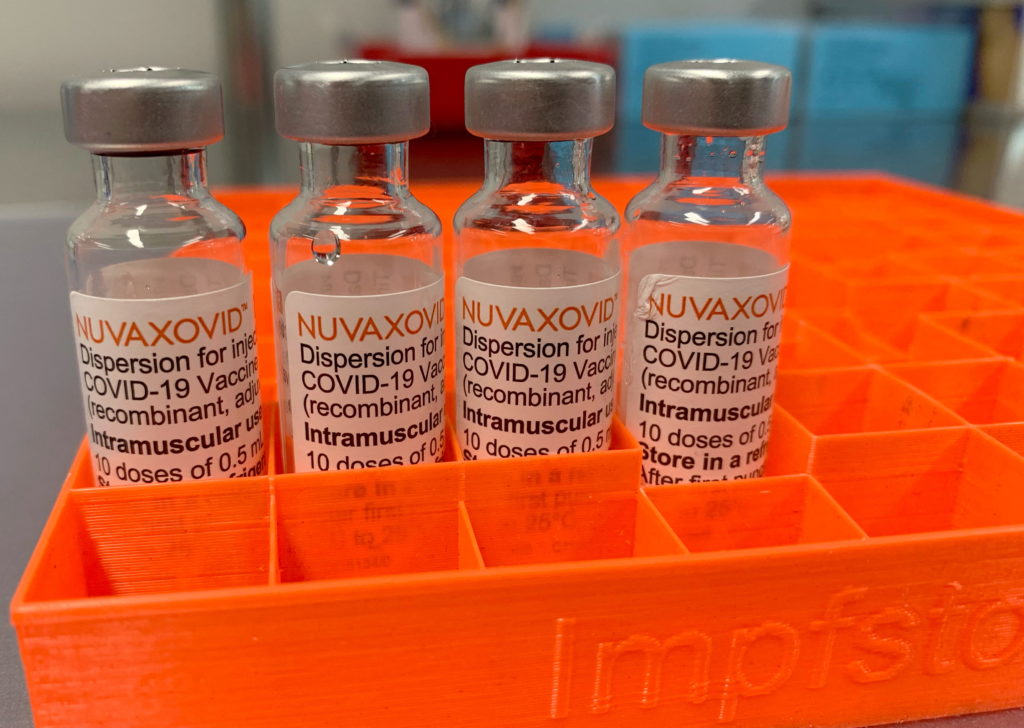
What is Nuvaxovid?
Nuvaxovid, developed by Novavax, is a protein subunit vaccine. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, it uses a different technology. It employs a piece of the virus's protein to trigger an immune response, making it an attractive option for some individuals hesitant about mRNA technology. This traditional approach may alleviate concerns for those seeking an alternative vaccination strategy. More information about protein subunit vaccines can be found on the .
FDA Approval: A Closer Look
The FDA's approval is a significant step, signifying that the vaccine has met stringent safety and efficacy standards. However, it's crucial to understand that this approval is not for all age groups. The FDA's authorization is currently limited to individuals 18 years of age and older. Further trials are needed to assess its efficacy and safety in younger populations.
Key Restrictions and Limitations:
- Age Restriction: As mentioned above, the current approval is solely for adults 18 and older. This limits its immediate applicability to a significant portion of the population. The lack of approval for younger age groups may impact broader vaccination campaigns.
- Efficacy Data: While the vaccine demonstrated efficacy in clinical trials, its effectiveness against newer variants remains an ongoing area of investigation. The evolving nature of the virus necessitates continued monitoring and potential adjustments to vaccination strategies.
- Supply and Distribution: The rollout of Nuvaxovid might face challenges related to supply and distribution. Given the existing vaccine landscape, the impact on vaccination rates remains to be seen. Logistics and accessibility will play a crucial role in determining the vaccine's overall effectiveness in curbing the pandemic.
- Potential Side Effects: Like all vaccines, Nuvaxovid carries the potential for side effects. While generally mild, individuals should be aware of potential reactions and seek medical attention if necessary. More information on potential side effects can be found on the .
The Future of Nuvaxovid:
The FDA's approval of Nuvaxovid offers a valuable addition to the existing COVID-19 vaccine portfolio. Its unique protein subunit technology provides an alternative for individuals who may prefer a non-mRNA vaccine. However, the restrictions surrounding age and ongoing research into its efficacy against new variants highlight the need for continued monitoring and further investigation. The long-term impact of Nuvaxovid on global vaccination efforts remains to be seen. Further clinical trials are expected to expand its use to younger age groups.
Conclusion:
The FDA's full approval of the Novavax COVID-19 vaccine is a positive development in the ongoing battle against the pandemic. However, it's essential to understand the limitations of this approval, particularly the age restriction. This understanding is critical for informed decision-making regarding vaccination strategies and public health initiatives. Stay updated with the latest information from reputable sources like the CDC and FDA for the most accurate and up-to-date details on COVID-19 vaccines.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Approval: Understanding The Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Ganassi Calls For Higher Standards For Team Penske To Protect Nascar
May 21, 2025
Ganassi Calls For Higher Standards For Team Penske To Protect Nascar
May 21, 2025 -
 Green Bay Packers Revise Controversial Tush Push Rule Proposal
May 21, 2025
Green Bay Packers Revise Controversial Tush Push Rule Proposal
May 21, 2025 -
 San Francisco 49ers Extend Linebacker Fred Warner For 63 Million
May 21, 2025
San Francisco 49ers Extend Linebacker Fred Warner For 63 Million
May 21, 2025 -
 Nfl Owners To Debate Revised Tush Push Ban Playoff Expansion And Flag Football
May 21, 2025
Nfl Owners To Debate Revised Tush Push Ban Playoff Expansion And Flag Football
May 21, 2025 -
 Christen Press Recovering After Angel City Fc Match Collapse Hospital Discharge Update
May 21, 2025
Christen Press Recovering After Angel City Fc Match Collapse Hospital Discharge Update
May 21, 2025
