Novavax COVID-19 Vaccine: FDA Approval Comes With Uncommon Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Approved by FDA, But With Caveats
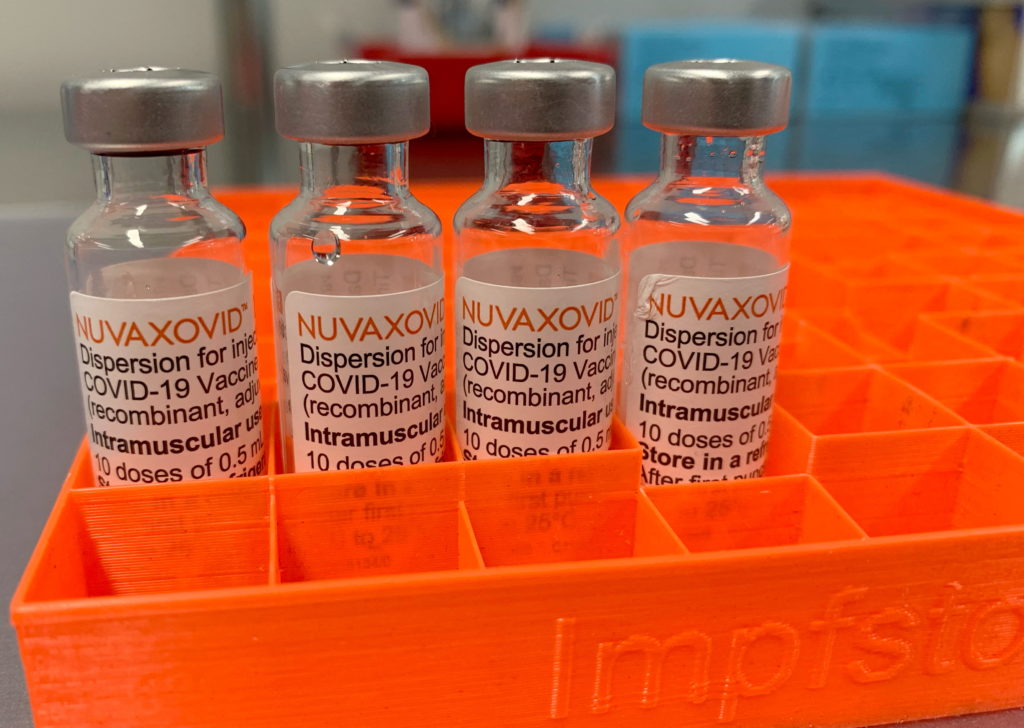
The FDA has finally granted approval to the Novavax COVID-19 vaccine, Nuvaxovid, offering another option for individuals seeking protection against the virus. However, this approval comes with some significant and uncommon usage restrictions that warrant careful consideration. This development marks a notable shift in the COVID-19 vaccine landscape, but understanding the limitations is crucial for both healthcare providers and the public.
Nuvaxovid: A Different Approach
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the adenovirus-vector vaccine from Johnson & Johnson, Nuvaxovid uses a more traditional protein subunit technology. This involves using harmless pieces of the virus to trigger an immune response. This approach has long been used in other vaccines and may appeal to individuals hesitant about mRNA technology. However, this established technology doesn't automatically translate to the same level of efficacy or ease of deployment as the newer mRNA counterparts.
FDA Approval: A Qualified Victory
While the FDA approval is undoubtedly a milestone, the agency has placed notable restrictions on its use. This isn't unprecedented – other vaccines have faced limitations based on their safety profile and efficacy data. However, the restrictions surrounding Nuvaxovid are particularly noteworthy.
Key Restrictions & Considerations:
- Limited Age Range: The FDA's approval is currently only for individuals aged 18 years and older. This contrasts with the broader age range covered by other approved COVID-19 vaccines. Further trials are needed to assess its safety and efficacy in younger populations.
- Specific Usage Recommendations: The FDA's authorization highlights specific circumstances where Nuvaxovid might be preferred. This might include individuals who have had adverse reactions to other vaccine types or those who prefer a protein-subunit vaccine. However, this preference should be discussed thoroughly with a healthcare professional.
- Efficacy Concerns: While the vaccine demonstrates efficacy against COVID-19, it might not provide the same level of protection compared to the mRNA vaccines, particularly against newer variants. This requires ongoing monitoring and potentially booster shots tailored to emerging variants.
- Supply Chain & Availability: The rollout of Nuvaxovid may face challenges initially due to potential manufacturing and distribution limitations. Its availability might vary significantly depending on location.
What This Means for You:
The approval of the Novavax vaccine expands the options available for COVID-19 vaccination. However, it's essential to consult with your doctor to determine if Nuvaxovid is the right choice for you, considering your individual health circumstances, risk factors, and access to other vaccine options. The restrictions imposed by the FDA highlight the need for a nuanced approach to vaccine selection, emphasizing informed decision-making based on a comprehensive discussion with healthcare professionals.
Looking Ahead:
The long-term efficacy and safety of Nuvaxovid will continue to be monitored. Further research will also focus on expanding its use to other age groups and assessing its effectiveness against future COVID-19 variants. The availability and uptake of this vaccine will play a significant role in achieving broader population immunity and mitigating the ongoing threat of COVID-19. Stay informed about updates from reliable sources like the CDC and FDA.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Always consult with a healthcare professional before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval Comes With Uncommon Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 The Fallout A J Perez On The Brett Favre Documentary And Subsequent Threats
May 20, 2025
The Fallout A J Perez On The Brett Favre Documentary And Subsequent Threats
May 20, 2025 -
 Japans Concession Reduced Not Eliminated Us Tariffs
May 20, 2025
Japans Concession Reduced Not Eliminated Us Tariffs
May 20, 2025 -
 Oklahoma City Thunder Defeat Denver Nuggets Heading To West Finals
May 20, 2025
Oklahoma City Thunder Defeat Denver Nuggets Heading To West Finals
May 20, 2025 -
 Mma Fans Erupt Jon Jones Latest Comments On Tom Aspinall Cause A Stir
May 20, 2025
Mma Fans Erupt Jon Jones Latest Comments On Tom Aspinall Cause A Stir
May 20, 2025 -
 Maple Leafs Fall To Panthers In Decisive Game 7 Matchup
May 20, 2025
Maple Leafs Fall To Panthers In Decisive Game 7 Matchup
May 20, 2025
