Novavax COVID-19 Vaccine: FDA Approval And Usage Constraints Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval and Usage Constraints Explained
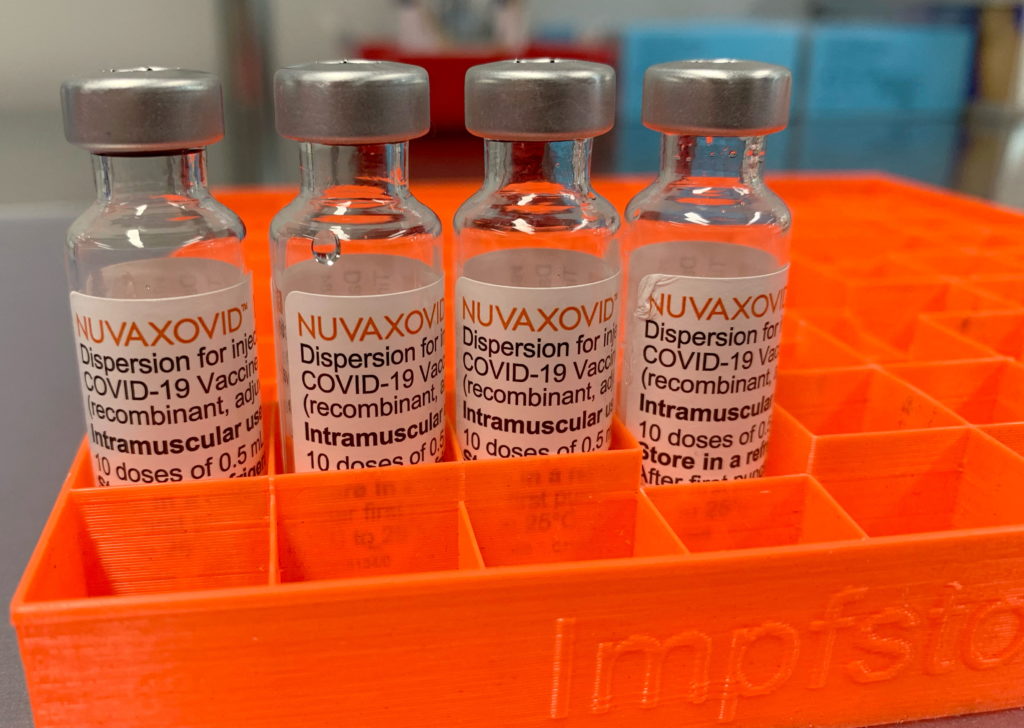
The Novavax COVID-19 vaccine, marketed as Nuvaxovid, has garnered significant attention since its FDA authorization. While offering a different technology compared to mRNA vaccines, its path to widespread use has been somewhat complex. This article will clarify the FDA approval process, explore the vaccine's mechanism of action, and delve into the specific usage constraints that have limited its broader adoption.
Understanding Novavax's Technology: Unlike the Pfizer-BioNTech and Moderna vaccines which utilize mRNA technology, Novavax employs a more traditional protein subunit approach. This involves using harmless pieces of the virus (spike proteins) to trigger an immune response. This method has been used for decades in other vaccines, potentially making it more familiar and appealing to some individuals hesitant about newer technologies. However, this established method also means a more complex manufacturing process.
FDA Approval and Authorization: The Novavax vaccine received Emergency Use Authorization (EUA) from the FDA in July 2022 for individuals 18 years and older. This authorization, while a crucial step, differed from the full licensure granted to other COVID-19 vaccines. The EUA signified that the vaccine met certain safety and effectiveness standards during a period of public health emergency. A subsequent full licensure was granted later, solidifying its place in the arsenal of available COVID-19 vaccines. [Link to FDA website regarding Novavax approval]
Usage Constraints and Limitations: Despite FDA approval, the Novavax vaccine hasn't achieved the same level of widespread adoption as its mRNA counterparts. Several factors contribute to this:
- Late Entry into the Market: By the time Novavax received authorization, other vaccines had already established distribution networks and widespread public familiarity. This late entry significantly impacted its market penetration.
- Lower Demand: With high vaccination rates already achieved using mRNA vaccines, the demand for a new vaccine, even with a different technology, was comparatively lower.
- Production Challenges: Manufacturing challenges initially hampered the scale of production, further limiting its availability.
- Efficacy Data: While effective, some studies showed slightly lower efficacy compared to mRNA vaccines, though still providing significant protection against severe illness. [Link to relevant study on Novavax efficacy]
- Specific Age Groups: Initially, the authorization was for adults only. Expansion to younger age groups might increase its usage, but currently, data on efficacy and safety for these age groups are still being evaluated.
Who Should Consider the Novavax Vaccine?
The Novavax vaccine presents a viable option for individuals who:
- Prefer a protein subunit vaccine: Those who are hesitant about mRNA technology might find this a more acceptable alternative.
- Have experienced side effects with other vaccines: While individual reactions vary, some individuals may find the side effects associated with the Novavax vaccine to be milder than those experienced with mRNA vaccines. (Always consult with a healthcare professional before making any vaccination decisions.)
- Have specific concerns about the other available vaccines: This decision should always be made in consultation with a doctor, who can assess individual health needs and risks.
Conclusion: The Novavax COVID-19 vaccine offers a valuable alternative within the landscape of available COVID-19 vaccines. While its usage has been constrained by various factors, its FDA approval and unique technological approach provide a choice for individuals seeking a different vaccination strategy. Understanding these constraints and considering individual needs will help ensure informed decision-making regarding COVID-19 vaccination. Remember to consult your healthcare provider for personalized advice.
Call to Action: Stay informed about the latest updates on COVID-19 vaccines and consult with your doctor to determine the best vaccination strategy for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval And Usage Constraints Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Thunders Triumph Conquering Nerves To Defeat Nuggets And Reach West Finals
May 20, 2025
Thunders Triumph Conquering Nerves To Defeat Nuggets And Reach West Finals
May 20, 2025 -
 Journalism Secures Preakness Victory Following Close Derby Call
May 20, 2025
Journalism Secures Preakness Victory Following Close Derby Call
May 20, 2025 -
 Inter Miamis Future Messi Offers Encouragement In Rare Interview
May 20, 2025
Inter Miamis Future Messi Offers Encouragement In Rare Interview
May 20, 2025 -
 From F1 To Indy Car Shwartzmans Unexpected Indy 500 Pole Victory
May 20, 2025
From F1 To Indy Car Shwartzmans Unexpected Indy 500 Pole Victory
May 20, 2025 -
 Jon Jones I M Done Message Sparks Debate Ufcs Next Heavyweight Contender
May 20, 2025
Jon Jones I M Done Message Sparks Debate Ufcs Next Heavyweight Contender
May 20, 2025
