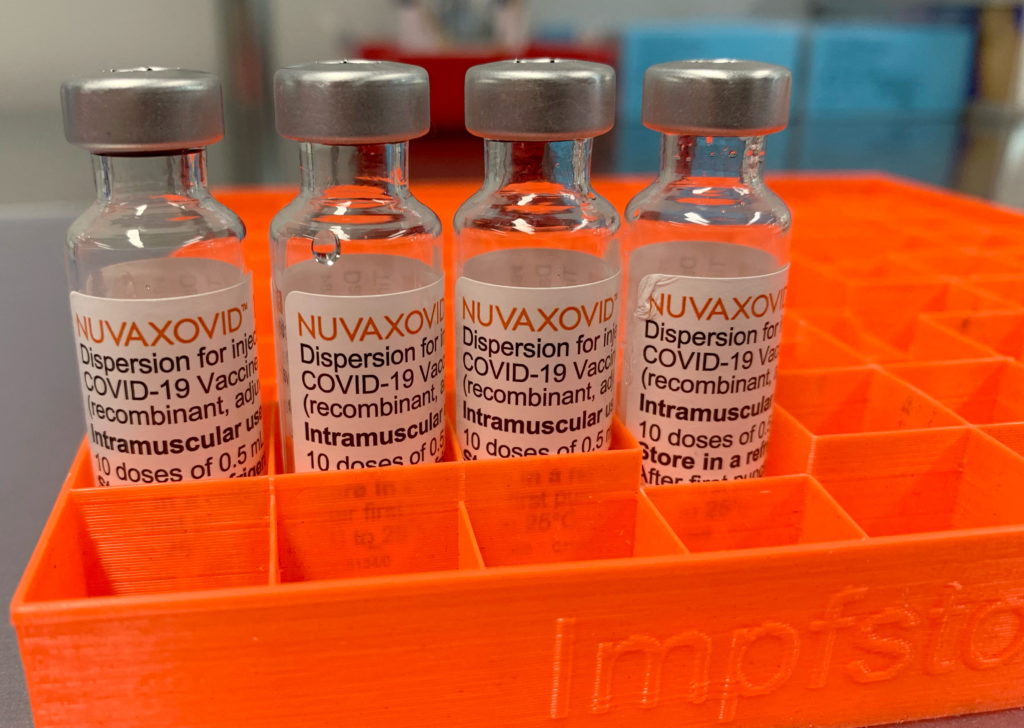
Novavax COVID-19 Vaccine Approved By FDA With Unusual Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Approved by FDA, but with Unusual Limitations
The FDA has finally granted approval to the Novavax COVID-19 vaccine, Nuvaxovid, but its authorization comes with significant caveats, raising questions about its market impact and future use. This marks a significant development in the fight against COVID-19, but the unusual limitations attached to the approval signal a more complex story than initially meets the eye.
What Makes Novavax's Approval Unusual?
Unlike the Pfizer-BioNTech and Moderna mRNA vaccines, which received Emergency Use Authorization (EUA) and later full FDA approval based on extensive real-world data and widespread usage, Novavax's approval relies on a more limited dataset. This has led to several unusual limitations:
-
Age Restrictions: The FDA's approval is currently limited to individuals 18 years of age and older. This contrasts sharply with the mRNA vaccines, which are authorized for use in younger age groups. Further trials are necessary to expand the age range for Novavax's vaccine.
-
Limited Supply: The FDA approval doesn't guarantee immediate widespread availability. The production and distribution capacity for Nuvaxovid remains a concern, potentially limiting its accessibility in the near term. This contrasts with the readily available mRNA vaccines.
-
Conditional Approval: The approval is conditional upon Novavax meeting certain post-market requirements, including ongoing monitoring of safety and effectiveness. This suggests a higher level of scrutiny compared to the established vaccines.
-
Specific Data Requirements: The FDA's approval is predicated on specific data requirements regarding safety and efficacy that Novavax must continue to meet. Failure to do so could result in the withdrawal of approval.
Why the Differences?
The differences in the approval process stem from several factors:
-
Delayed Development: Novavax's vaccine entered the clinical trial process later than its mRNA counterparts, resulting in a smaller and potentially less diverse dataset for review.
-
Different Technology: Novavax uses a protein-based technology, which, while considered safer by some, may have presented unique challenges in the development and regulatory approval processes. The established safety profiles of the mRNA vaccines contributed to their swifter approvals.
-
Shifting Pandemic Landscape: The COVID-19 pandemic landscape shifted significantly while Novavax was undergoing development and testing. The emergence of variants and widespread vaccination efforts affected the relevance of the clinical trial data.
What Does This Mean for the Future of COVID-19 Vaccination?
The approval of the Novavax vaccine offers a valuable alternative for individuals who may have concerns about mRNA technology. However, the limitations attached to the approval highlight the challenges faced in bringing new vaccines to market during a rapidly evolving public health crisis.
The limited supply and age restrictions likely mean Nuvaxovid won't significantly disrupt the established vaccine landscape. However, its approval demonstrates the ongoing need for vaccine diversity and the importance of continuous research and development in the fight against infectious diseases.
Further Research and Monitoring are Crucial:
The FDA’s conditional approval emphasizes the need for continued monitoring of the Novavax vaccine's safety and efficacy in real-world settings. Long-term data collection will be crucial in fully understanding its potential contribution to COVID-19 vaccination strategies. It will be important to track its effectiveness against emerging variants and to further refine its deployment based on evolving needs.
Call to Action: Stay informed about updates on the Novavax vaccine's availability and safety profile through reliable sources like the FDA and the CDC websites. Discuss vaccine options with your healthcare provider to determine the best choice for your individual needs.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Approved By FDA With Unusual Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Nfl 2023 Postseason The Underdogs And Fringe Teams Most Likely To Qualify
May 20, 2025
Nfl 2023 Postseason The Underdogs And Fringe Teams Most Likely To Qualify
May 20, 2025 -
 Grief And Grit Scheifeles Performance After Devastating Loss
May 20, 2025
Grief And Grit Scheifeles Performance After Devastating Loss
May 20, 2025 -
 Global Collaboration Needed To Enhance Safety And Respect In Balis Tourism Sector
May 20, 2025
Global Collaboration Needed To Enhance Safety And Respect In Balis Tourism Sector
May 20, 2025 -
 Doubt Cast On Marcus Armstrongs Indianapolis 500 Participation After Crash
May 20, 2025
Doubt Cast On Marcus Armstrongs Indianapolis 500 Participation After Crash
May 20, 2025 -
 Beyond The Studio 5 Shows For Fans
May 20, 2025
Beyond The Studio 5 Shows For Fans
May 20, 2025
