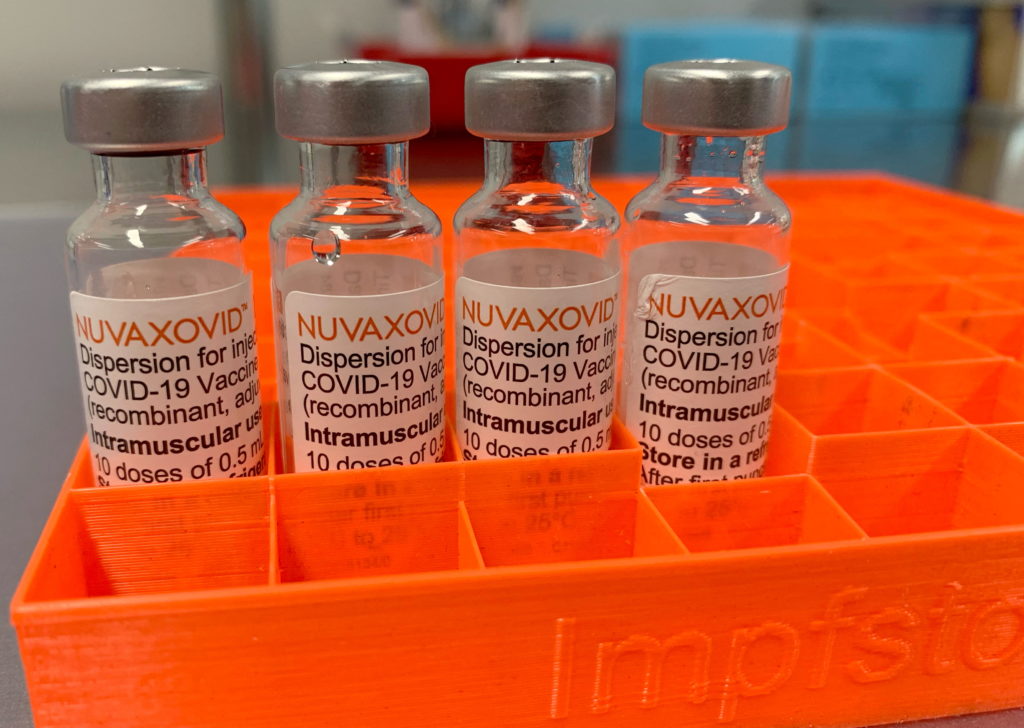
Novavax COVID-19 Vaccine Approved By FDA, But With Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Approved by FDA, But With Limitations
The FDA grants approval to Novavax's COVID-19 vaccine, Nuvaxovid, but with caveats impacting its widespread use.
The FDA's approval of Novavax's COVID-19 vaccine, Nuvaxovid, marks a significant step in the fight against the pandemic. However, the approval comes with limitations that might restrict its immediate impact on vaccination efforts. This protein-subunit vaccine, a different technology than the mRNA vaccines from Pfizer-BioNTech and Moderna, offers a potentially valuable alternative for those hesitant about mRNA technology. But its late arrival and conditional approval raise questions about its practical application.
Understanding the FDA's Approval:
The FDA's approval is for individuals 18 years of age and older. This is a significant difference from the mRNA vaccines, which are authorized for use in younger age groups. The approval is also conditional, meaning ongoing monitoring and data collection are required to confirm its long-term safety and effectiveness. This conditional nature stems from the relatively smaller trial size compared to the mRNA vaccine trials, leading to a less comprehensive data set.
Why the Limitations?
Several factors contribute to the limitations placed on Nuvaxovid's approval:
- Late Entry into the Market: The vaccine's development and approval process lagged behind those of the mRNA vaccines. By the time it received approval, widespread vaccination campaigns were already underway, diminishing the immediate need for another vaccine.
- Production Challenges: Reports suggest that production challenges have hampered the vaccine's rollout. This means that even with FDA approval, sufficient doses might not be readily available to meet the demand.
- Efficacy Concerns: While the vaccine has shown efficacy against COVID-19, its effectiveness against newer variants, such as Omicron and its subvariants, might be lower compared to the updated mRNA boosters. This is a critical aspect that needs continuous evaluation.
- Safety Profile: While generally safe, the FDA approval necessitates continued monitoring of the vaccine's safety profile, particularly for rare adverse events. This is standard procedure for all new vaccines but warrants special attention given the time sensitivity of the pandemic response.
Who Might Benefit from the Novavax Vaccine?
Despite the limitations, the Novavax vaccine offers a valuable alternative for specific populations. Individuals who have concerns about mRNA technology or have experienced adverse reactions to other COVID-19 vaccines might find Nuvaxovid a suitable option. This presents a crucial choice for those seeking a different vaccine platform.
The Future of Nuvaxovid:
The long-term success of Nuvaxovid hinges on addressing production challenges and demonstrating sustained efficacy against emerging variants. Further research and real-world data will be crucial in determining its overall impact on global vaccination efforts. The FDA's continued monitoring will also play a vital role in ensuring its safe and effective use. The future remains uncertain, but the availability of a protein-subunit vaccine provides a valuable option within the broader context of the pandemic response.
Call to Action:
Consult your physician to determine if the Novavax COVID-19 vaccine is the right choice for you. Staying informed about vaccine updates and adhering to public health guidelines remain crucial in managing the ongoing pandemic. For more information on COVID-19 vaccines, visit the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Approved By FDA, But With Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Toledo Mud Hens Defeat Scranton Wilkes Barre Rail Riders In Dominant Performance
May 20, 2025
Toledo Mud Hens Defeat Scranton Wilkes Barre Rail Riders In Dominant Performance
May 20, 2025 -
 200 Million Ethereum Investment Boom Follows Successful Pectra Upgrade
May 20, 2025
200 Million Ethereum Investment Boom Follows Successful Pectra Upgrade
May 20, 2025 -
 Institutional Money Fuels Bitcoin Etf Growth 5 Billion And Counting
May 20, 2025
Institutional Money Fuels Bitcoin Etf Growth 5 Billion And Counting
May 20, 2025 -
 Stock Market Rally S And P 500s 6 Day Winning Streak Dow And Nasdaq Gains
May 20, 2025
Stock Market Rally S And P 500s 6 Day Winning Streak Dow And Nasdaq Gains
May 20, 2025 -
 Trae Youngs Public Praise For Thunder Fans A Sharp Contrast To Knicks Rivalry
May 20, 2025
Trae Youngs Public Praise For Thunder Fans A Sharp Contrast To Knicks Rivalry
May 20, 2025
