Limited FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
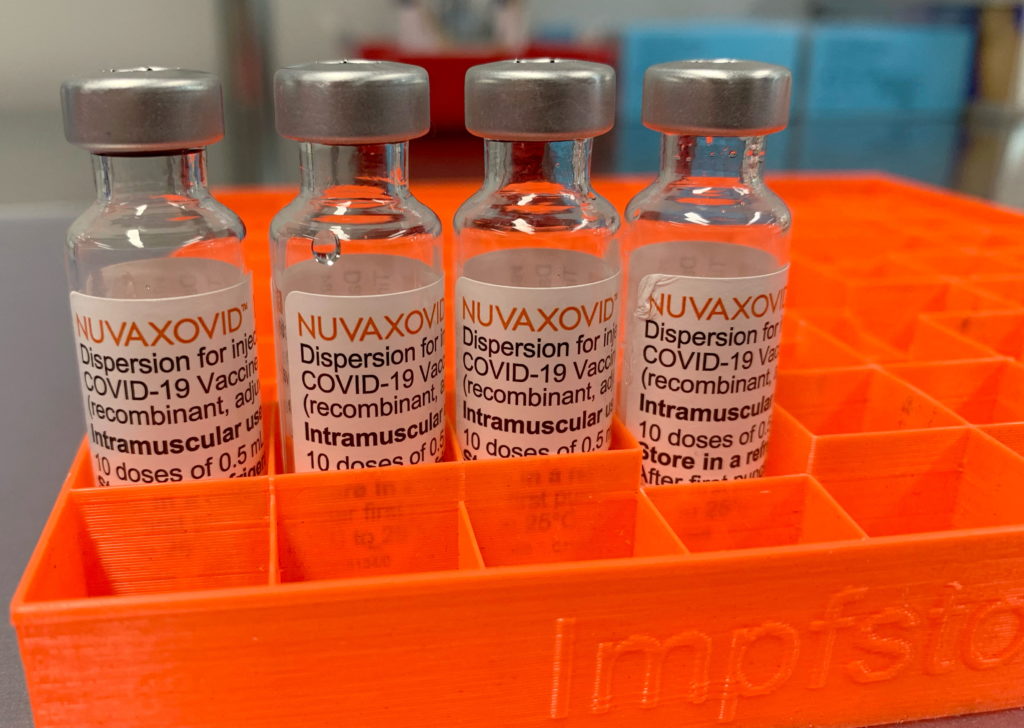
Limited FDA Approval: Understanding the Novavax COVID-19 Vaccine Restrictions
The Novavax COVID-19 vaccine, marketed as Nuvaxovid, finally gained FDA approval in July 2023. However, this approval wasn't a complete green light. Unlike the widely used mRNA vaccines, Nuvaxovid's authorization carries significant restrictions, leaving many wondering about its place in the current COVID-19 landscape. This article will clarify the limitations surrounding Novavax's approval and explore why these restrictions exist.
What is the Novavax COVID-19 Vaccine?
Nuvaxovid is a protein subunit vaccine, a technology that's been around for decades and is used in vaccines against other diseases like Hepatitis B. Unlike mRNA vaccines that use messenger RNA to instruct cells to produce a viral protein, Novavax utilizes purified fragments of the SARS-CoV-2 virus's spike protein. This protein triggers an immune response, teaching the body to recognize and fight the virus. This traditional approach is seen by some as more familiar and potentially less anxiety-inducing than the newer mRNA technology.
Understanding the FDA's Limited Approval
While the FDA granted approval, it's crucial to understand the limitations. The approval is specifically for individuals 18 years of age and older. This differs from other authorized vaccines which are available for younger age groups. This limitation is likely due to the ongoing data collection and analysis required to ensure the vaccine's safety and efficacy across all age demographics.
Furthermore, the approval is not for primary vaccination series. Currently, Novavax is primarily authorized for use as a booster shot for individuals who have already completed a primary vaccination series with another authorized COVID-19 vaccine. This strategy reflects the current understanding of COVID-19 immunity and the vaccine's role in maintaining protection.
Why the Restrictions?
The reasons behind the limited approval are multifaceted:
- Clinical Trial Data: The FDA approval was based on clinical trial data, and while the results demonstrated efficacy and safety, they may not be as extensive or conclusive as those for other vaccines, particularly concerning younger age groups. More research is needed before expanding authorization.
- Manufacturing Challenges: Early production challenges contributed to delays in the vaccine's rollout. These hurdles may have also impacted the availability of data necessary for a broader approval.
- Evolving Landscape: The COVID-19 landscape is constantly changing with new variants emerging. The FDA's cautious approach allows for further monitoring of the vaccine's effectiveness against these new variants.
What Does This Mean for You?
If you are 18 years or older and have already received a primary COVID-19 vaccination series, Novavax may be an option for your booster shot. However, it's crucial to discuss your vaccination options with your healthcare provider. They can help you determine the most appropriate vaccine based on your individual health history and risk factors. This personalized approach remains essential in maximizing the benefits and minimizing the risks associated with COVID-19 vaccination.
Staying Informed About COVID-19 Vaccines
The information presented here is for educational purposes and should not be considered medical advice. For the most up-to-date information on COVID-19 vaccines, including any changes in authorization or recommendations, consult the CDC website [link to CDC website] and your healthcare provider. Staying informed is crucial in navigating this evolving public health situation. Remember to always consult a medical professional before making decisions about your health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Late Drama Leeds Rhinos Defeat Hull Fc 18 16 In Intense Super League Match
May 20, 2025
Late Drama Leeds Rhinos Defeat Hull Fc 18 16 In Intense Super League Match
May 20, 2025 -
 Always Kept It Real Jamie Lee Curtiss Honest Assessment Of Lindsay Lohan
May 20, 2025
Always Kept It Real Jamie Lee Curtiss Honest Assessment Of Lindsay Lohan
May 20, 2025 -
 Post Pectra Upgrade Ethereum Funds Attract 200 Million In Investor Capital
May 20, 2025
Post Pectra Upgrade Ethereum Funds Attract 200 Million In Investor Capital
May 20, 2025 -
 2025 Nba Championship Early Odds Point To Thunder And Knicks Dominance
May 20, 2025
2025 Nba Championship Early Odds Point To Thunder And Knicks Dominance
May 20, 2025 -
 Dallas Stars Punch Ticket To Western Conference Finals For Third Straight Season
May 20, 2025
Dallas Stars Punch Ticket To Western Conference Finals For Third Straight Season
May 20, 2025
