Limited FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Limited FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions
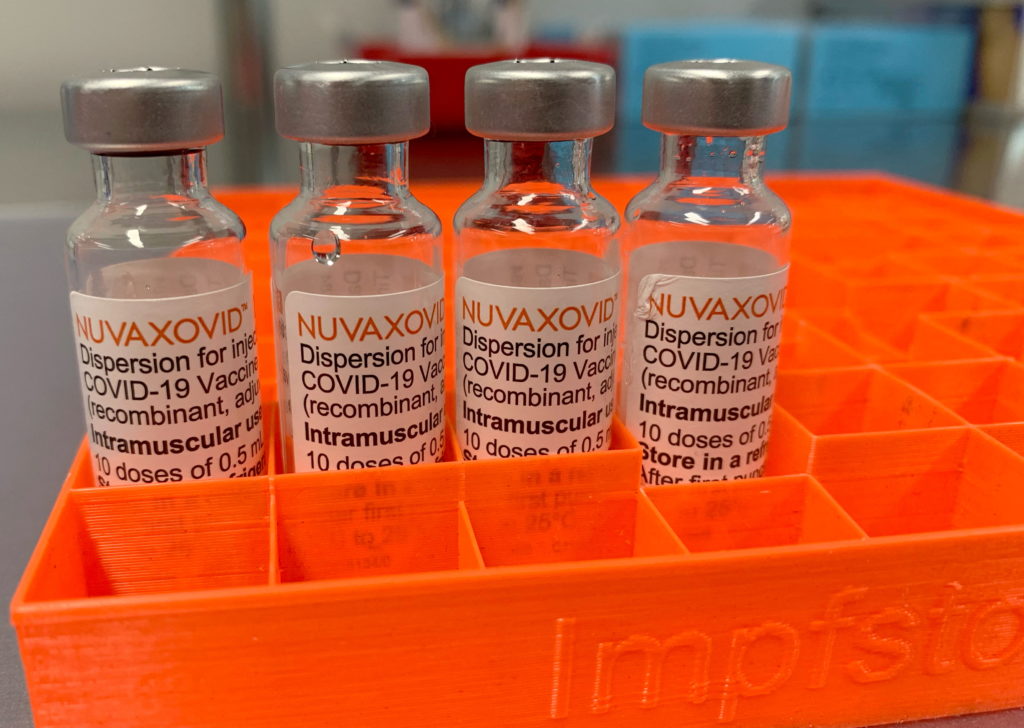
The Novavax COVID-19 vaccine, Nuvaxovid, has received limited approval from the FDA, raising questions about its future role in the ongoing pandemic response. While its arrival offers an alternative to mRNA vaccines, significant usage restrictions cast a shadow on its widespread adoption. This article delves into the specifics of the FDA's decision and explores the implications for public health.
FDA Approval with Caveats: Understanding the Nuances
The FDA granted Emergency Use Authorization (EUA) for Nuvaxovid, but this authorization is not without limitations. Unlike the widely used Pfizer-BioNTech and Moderna vaccines, Nuvaxovid's approval is specifically for individuals 18 years of age and older. This exclusion of younger age groups immediately restricts its potential market share. Furthermore, the FDA's approval emphasizes the need for careful monitoring of potential side effects, highlighting a key difference from the established mRNA vaccines which have a more extensive safety profile at this point.
This limited EUA reflects a cautious approach by the FDA, prioritizing safety and efficacy data. The agency’s decision underscores the rigorous process involved in evaluating and approving new vaccines, especially in the context of a rapidly evolving public health crisis.
Why the Restrictions? Analyzing the Data
The FDA's decision to limit the EUA is likely based on a number of factors. While Novavax demonstrated efficacy in clinical trials, the data might not be as robust or extensive as that available for the mRNA vaccines. This could involve fewer participants in the trials, shorter follow-up periods, or less comprehensive data on specific subgroups. The relatively late entry into the market also means less real-world usage data is available for comparison.
Additionally, the emergence of new COVID-19 variants with varying levels of vaccine resistance could have influenced the FDA's decision. The efficacy of Nuvaxovid against these newer variants requires further investigation and monitoring.
Novavax's Place in the COVID-19 Vaccination Landscape
Despite the limitations, Nuvaxovid's arrival presents a valuable alternative for certain individuals. Those hesitant about mRNA technology may find the protein-based Novavax vaccine more appealing. This offers a crucial choice for those seeking vaccination but wary of the mRNA platforms. The protein-based technology has been used in vaccines for many years, potentially increasing acceptance among vaccine-hesitant populations.
However, the restricted usage limits its overall impact on the pandemic response. The limited availability and age restrictions reduce its potential contribution to achieving herd immunity and minimizing the spread of COVID-19.
Looking Ahead: Future of Novavax and COVID-19 Vaccination
The long-term prospects of Nuvaxovid remain uncertain. Further clinical trials and ongoing monitoring will be crucial in determining its efficacy against emerging variants and its long-term safety profile. The FDA may expand the EUA to include younger age groups depending on the results of these ongoing studies. The vaccine's effectiveness in boosting immunity in individuals who have already received other COVID-19 vaccines is also an area requiring further research.
The journey of the Novavax vaccine highlights the complexities of vaccine development and deployment during a public health emergency. While offering a different technological approach, its current limited availability and restrictions underscore the ongoing need for continuous monitoring and adaptation in the fight against COVID-19. The FDA's measured approach underscores its commitment to ensuring vaccine safety and effectiveness, a priority that benefits public trust and the overall effectiveness of the vaccination campaign.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Chip Ganassi Calls For Higher Standards At Team Penske To Protect Nascar
May 21, 2025
Chip Ganassi Calls For Higher Standards At Team Penske To Protect Nascar
May 21, 2025 -
 Super Regional Bound Libertys Upset Victory Over Texas A And M
May 21, 2025
Super Regional Bound Libertys Upset Victory Over Texas A And M
May 21, 2025 -
 Which Bubble Teams Will Make The 2023 Nfl Playoffs A Realistic Assessment
May 21, 2025
Which Bubble Teams Will Make The 2023 Nfl Playoffs A Realistic Assessment
May 21, 2025 -
 Nfl Rule Change Green Bay Packers Revised Tush Push Proposal Explained
May 21, 2025
Nfl Rule Change Green Bay Packers Revised Tush Push Proposal Explained
May 21, 2025 -
 Pga Championship Heartbreak Jon Rahms Resilience In Defeat
May 21, 2025
Pga Championship Heartbreak Jon Rahms Resilience In Defeat
May 21, 2025
