Limited FDA Approval For Novavax COVID-19 Vaccine: What You Need To Know
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
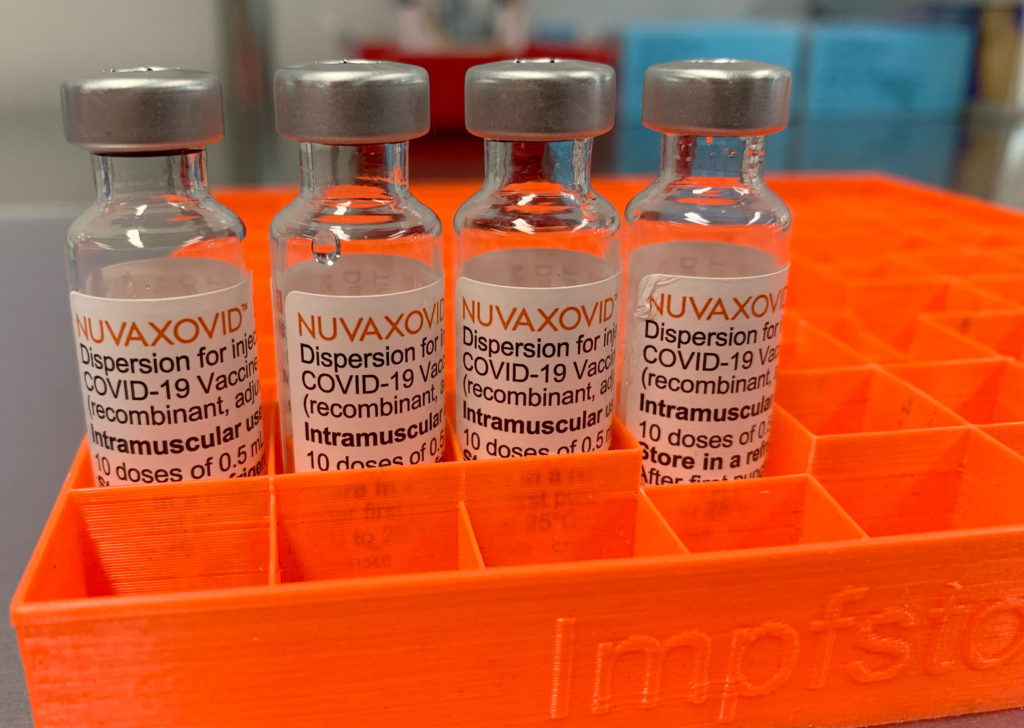
Limited FDA Approval for Novavax COVID-19 Vaccine: What You Need to Know
The Novavax COVID-19 vaccine, marketed as Nuvaxovid, has received limited approval from the FDA, marking a significant development in the ongoing fight against the pandemic. This approval, however, comes with nuances that are crucial for individuals considering this vaccine option. Let's delve into the details and understand what this means for you.
What is the FDA's Approval Status?
The FDA granted Emergency Use Authorization (EUA) for Nuvaxovid in July 2023. While this isn't full licensure, it signifies that the agency has determined the vaccine's benefits outweigh its risks for individuals 12 years of age and older. It's important to note that this authorization is distinct from the full Biologics License Application (BLA) approval received by other COVID-19 vaccines. This limited approval means the vaccine is available for use, but ongoing monitoring and further data collection are still required.
How Does Novavax Differ from Other COVID-19 Vaccines?
Unlike the mRNA vaccines (Pfizer-BioNTech and Moderna) and the viral vector vaccine (Johnson & Johnson), Novavax utilizes a protein-subunit technology. This approach uses harmless pieces of the virus to trigger an immune response. This difference may appeal to individuals hesitant about mRNA technology. It's crucial, however, to understand that while the technology differs, the goal remains the same: to build immunity against COVID-19. Efficacy and safety profiles should be considered individually, and discussions with your healthcare provider are vital.
What are the Potential Benefits and Risks?
Benefits:
- Different Technology: The protein-subunit platform may appeal to those wary of mRNA vaccines.
- Strong Efficacy: Clinical trials have demonstrated strong efficacy against COVID-19, particularly against severe disease. [Link to relevant clinical trial data]
- Potential for Broad Immunity: Some studies suggest potential for broad immunity against various COVID-19 variants. [Link to supporting research]
Risks:
- Side Effects: Like other vaccines, Novavax can cause side effects, ranging from mild (pain at the injection site, fatigue) to more serious (rare allergic reactions). [Link to FDA side effect information]
- Limited Long-Term Data: The long-term effects of Novavax are still under investigation due to the relatively recent introduction of the vaccine.
- Availability: Availability may vary depending on location.
Who Should Consider Novavax?
Individuals 12 years and older who are eligible for a COVID-19 vaccine are generally considered eligible for Novavax. However, individuals with specific allergies or health concerns should consult their healthcare provider to discuss the suitability of this vaccine. This is particularly important given the limited long-term data currently available.
Where Can I Get the Novavax Vaccine?
The availability of the Novavax vaccine varies by region. Check with your healthcare provider or local health department to find vaccination sites offering Nuvaxovid. [Link to vaccine finder tool - if available]
Conclusion:
The limited FDA approval of the Novavax COVID-19 vaccine provides another option in the fight against the virus. While its protein-subunit technology offers a different approach, understanding both its potential benefits and risks is crucial. Consult your healthcare provider to make an informed decision about which COVID-19 vaccine is right for you. Staying informed about the latest updates from the FDA and CDC is also essential. Remember, vaccination remains a vital tool in protecting yourself and your community.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval For Novavax COVID-19 Vaccine: What You Need To Know. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Relive The Excitement Nascar All Star Race At North Wilkesboro Speedway
May 20, 2025
Relive The Excitement Nascar All Star Race At North Wilkesboro Speedway
May 20, 2025 -
 No Oval Experience No Problem Shwartzmans Stunning Indy 500 Pole Victory
May 20, 2025
No Oval Experience No Problem Shwartzmans Stunning Indy 500 Pole Victory
May 20, 2025 -
 Twins Correa Buxton Suffer Concussions Placed On 7 Day Injured List
May 20, 2025
Twins Correa Buxton Suffer Concussions Placed On 7 Day Injured List
May 20, 2025 -
 Lost Pets A Memorial For Victims Of Funeral Home Cremains Mishandling
May 20, 2025
Lost Pets A Memorial For Victims Of Funeral Home Cremains Mishandling
May 20, 2025 -
 Ethereum Sees Massive 200 Million Investment Boost After Pectra Upgrade
May 20, 2025
Ethereum Sees Massive 200 Million Investment Boost After Pectra Upgrade
May 20, 2025
