Limited Approval: FDA Authorizes Novavax COVID-19 Vaccine Under Specific Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Limited Approval: FDA Authorizes Novavax COVID-19 Vaccine Under Specific Conditions
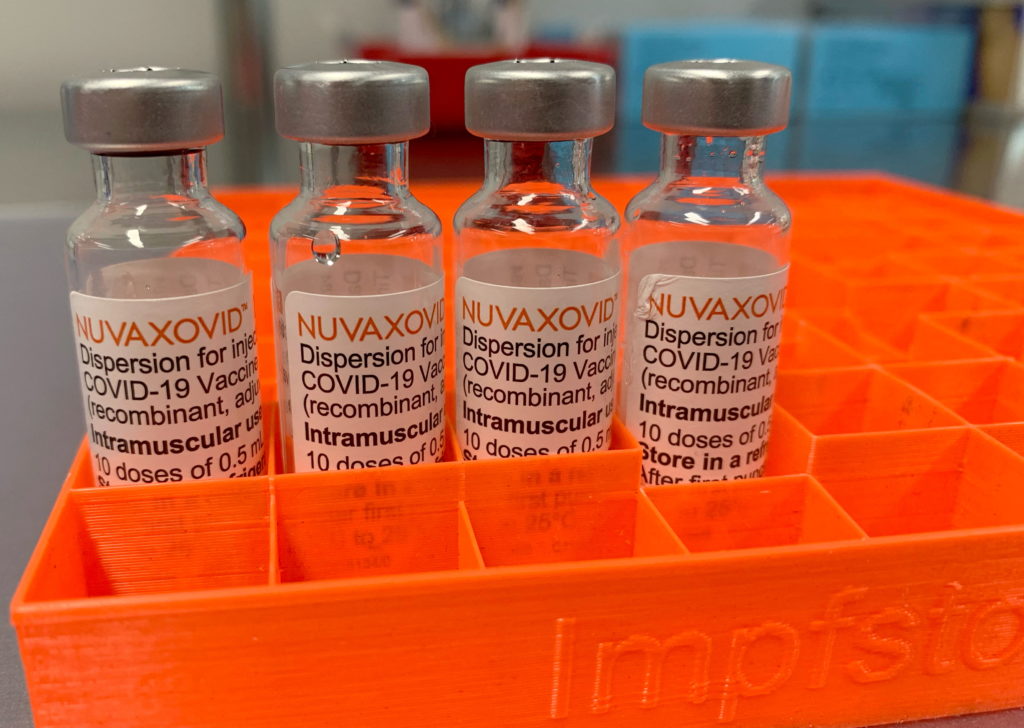
The FDA has granted limited approval to the Novavax COVID-19 vaccine, Nuvaxovid, but with significant caveats. This authorization, announced [Insert Date of Announcement], marks a significant development in the fight against the pandemic, offering a new vaccine option for those hesitant about mRNA technology. However, the conditional approval highlights ongoing concerns regarding the vaccine's efficacy and safety profile compared to its mRNA counterparts.
Understanding the FDA's Conditional Approval:
The FDA's authorization is not a full approval. Instead, it's granted under an Emergency Use Authorization (EUA) framework, meaning it's approved for use during the ongoing public health emergency. This differs from a full Biologics License Application (BLA) approval, which requires more extensive and long-term data on safety and effectiveness. The EUA is contingent upon Novavax meeting certain post-market surveillance requirements, including ongoing monitoring of safety data and continued efficacy studies.
What Makes Novavax Different?
Unlike the Pfizer-BioNTech and Moderna vaccines which utilize mRNA technology, Novavax's Nuvaxovid is a protein subunit vaccine. This means it uses a piece of the virus's protein to trigger an immune response, a technology that has been used for decades in other vaccines. This difference might appeal to individuals hesitant about mRNA vaccines due to concerns – often unfounded – about their novel technology.
Efficacy and Safety Concerns:
While the FDA has deemed Nuvaxovid safe and effective, its efficacy data trails behind the mRNA vaccines. Clinical trials showed a lower efficacy rate against infection compared to Pfizer-BioNTech and Moderna. Furthermore, some early concerns regarding adverse events, particularly myocarditis (inflammation of the heart muscle), are currently being investigated. The FDA will continue to closely monitor these reports. [Link to FDA Website on vaccine safety data].
Who Can Get the Novavax Vaccine?
Currently, the Novavax vaccine is authorized for individuals 18 years of age and older. The FDA’s decision is based on the available data from clinical trials. Future authorization for younger age groups might depend on further research and data analysis.
The Future of the Novavax Vaccine:
The conditional approval of the Novavax vaccine presents both opportunities and challenges. It offers an alternative for individuals wary of mRNA vaccines, potentially increasing vaccination rates. However, its lower efficacy and ongoing safety monitoring underline the need for continued vigilance and further research. The long-term impact of the vaccine remains to be seen, and its role in the overall COVID-19 vaccination strategy will depend on ongoing surveillance data and future clinical trials.
Call to Action:
Consult with your healthcare provider to discuss the Novavax vaccine and determine if it's the right choice for you. Remember to stay informed about the latest updates from trusted sources like the CDC and FDA. [Link to CDC website on COVID-19 vaccination].
Keywords: Novavax, COVID-19 vaccine, FDA approval, EUA, protein subunit vaccine, mRNA vaccine, vaccine efficacy, vaccine safety, Nuvaxovid, COVID-19 vaccination, public health emergency, Biologics License Application (BLA), post-market surveillance.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited Approval: FDA Authorizes Novavax COVID-19 Vaccine Under Specific Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 North Carolina Weather Alert Severe Storms And Heavy Rain Possible Overnight
May 21, 2025
North Carolina Weather Alert Severe Storms And Heavy Rain Possible Overnight
May 21, 2025 -
 Analysis The High Number Of Crashes During Indy 500 Practice
May 21, 2025
Analysis The High Number Of Crashes During Indy 500 Practice
May 21, 2025 -
 Exclusive Jamie Lee Curtis Reveals How She Stays Connected With Lindsay Lohan
May 21, 2025
Exclusive Jamie Lee Curtis Reveals How She Stays Connected With Lindsay Lohan
May 21, 2025 -
 High Expectations For Cavaliers A Must Win Season For Cleveland
May 21, 2025
High Expectations For Cavaliers A Must Win Season For Cleveland
May 21, 2025 -
 Game 7 Thunders Convincing Win Against Nuggets Ends Denvers Playoff Run
May 21, 2025
Game 7 Thunders Convincing Win Against Nuggets Ends Denvers Playoff Run
May 21, 2025
