FDA Greenlights Novavax COVID-19 Vaccine, But With Strict Usage Guidelines
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Greenlights Novavax COVID-19 Vaccine, but with Strict Usage Guidelines
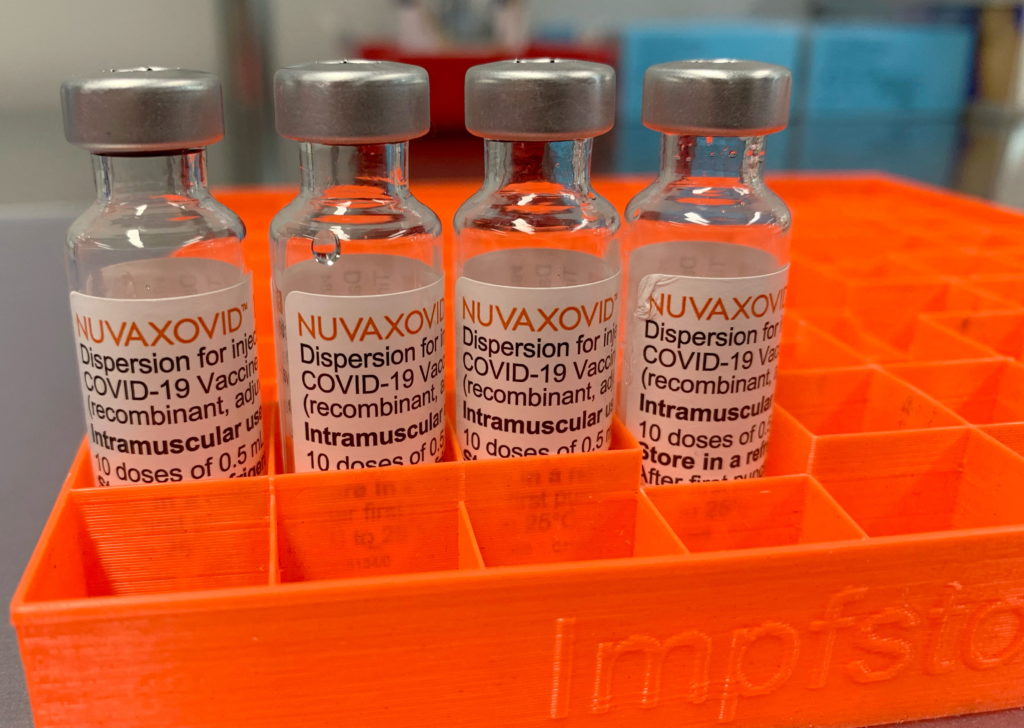
The FDA has finally granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, offering a new option for individuals hesitant about mRNA vaccines. However, this approval comes with strict usage guidelines, limiting its immediate impact on the broader vaccination campaign. This news marks a significant development in the ongoing fight against the pandemic, but understanding the limitations is crucial.
A Different Approach: Protein Subunit Technology
Unlike the Pfizer-BioNTech and Moderna vaccines utilizing mRNA technology, Novavax's vaccine employs a more traditional protein subunit approach. This method uses harmless pieces of the virus to trigger an immune response, potentially appealing to individuals concerned about mRNA's newer technology. This difference in technology might be a key factor for those seeking alternative COVID-19 vaccination options. Many people have been eagerly awaiting a non-mRNA vaccine, and this approval answers those calls.
The FDA's Cautious Approval: Key Restrictions and Usage Guidelines
While the authorization is positive news, the FDA has imposed specific limitations on Nuvaxovid's use. The vaccine is currently authorized only for individuals aged 18 and older. This contrasts with the wider age ranges covered by the mRNA vaccines. Furthermore, the FDA's authorization includes stringent guidelines regarding storage and handling, potentially impacting its widespread distribution and accessibility. These logistical challenges could hinder its immediate rollout.
Limited Supply and Distribution Challenges
The initial supply of Novavax's vaccine will likely be limited, presenting another hurdle to its rapid deployment. This constrained supply, combined with the strict handling requirements, could lead to initial distribution difficulties. Health officials will need to strategically allocate available doses to prioritize high-risk populations, which will be a focus moving forward.
Addressing Vaccine Hesitancy: A Potential Game Changer?
Some experts believe that the availability of a non-mRNA vaccine could help address vaccine hesitancy among certain populations. The different technology might persuade individuals who have been reluctant to receive the mRNA vaccines to get vaccinated. This could be a critical factor in increasing overall vaccination rates and community immunity. However, this remains to be seen, and further public health initiatives will be necessary to effectively communicate the benefits and safety profile of Nuvaxovid.
Looking Ahead: Monitoring Effectiveness and Safety
The FDA's approval is not the end of the story. Ongoing monitoring of the vaccine's effectiveness and safety profile will be vital. Post-market surveillance will track any adverse effects and assess the vaccine's long-term efficacy against emerging COVID-19 variants. This continuous evaluation is standard practice and will help refine vaccination strategies.
Conclusion: A Step Forward, but with Challenges Ahead
The FDA's approval of Novavax's COVID-19 vaccine represents a significant step forward in the fight against the pandemic. However, the strict usage guidelines and logistical challenges pose considerable obstacles to its widespread adoption. The vaccine's potential to address vaccine hesitancy remains to be seen, but its introduction undeniably provides a valuable new tool in our arsenal against COVID-19. Further information and updates will be released by the CDC and FDA in the coming weeks. Stay informed and consult with your healthcare provider to determine the best vaccination strategy for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Greenlights Novavax COVID-19 Vaccine, But With Strict Usage Guidelines. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Celtics Championship Window Assessing The Impact Of The Upcoming Offseason
May 21, 2025
Celtics Championship Window Assessing The Impact Of The Upcoming Offseason
May 21, 2025 -
 From Formula 2 To Indy 500 Pole Robert Shwartzmans Unexpected Victory
May 21, 2025
From Formula 2 To Indy 500 Pole Robert Shwartzmans Unexpected Victory
May 21, 2025 -
 Jamie Lee Curtis Opens Up About Her Bond With Lindsay Lohan Post Freaky Friday
May 21, 2025
Jamie Lee Curtis Opens Up About Her Bond With Lindsay Lohan Post Freaky Friday
May 21, 2025 -
 Overnight Storm Forecast For Charlotte Prepare For Cooler Temperatures
May 21, 2025
Overnight Storm Forecast For Charlotte Prepare For Cooler Temperatures
May 21, 2025 -
 Referee Rooneys Post Suspension Return Eyes On The Playoffs
May 21, 2025
Referee Rooneys Post Suspension Return Eyes On The Playoffs
May 21, 2025
