FDA Greenlights Novavax COVID-19 Vaccine, But With Strict Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Greenlights Novavax COVID-19 Vaccine, but with Strict Conditions
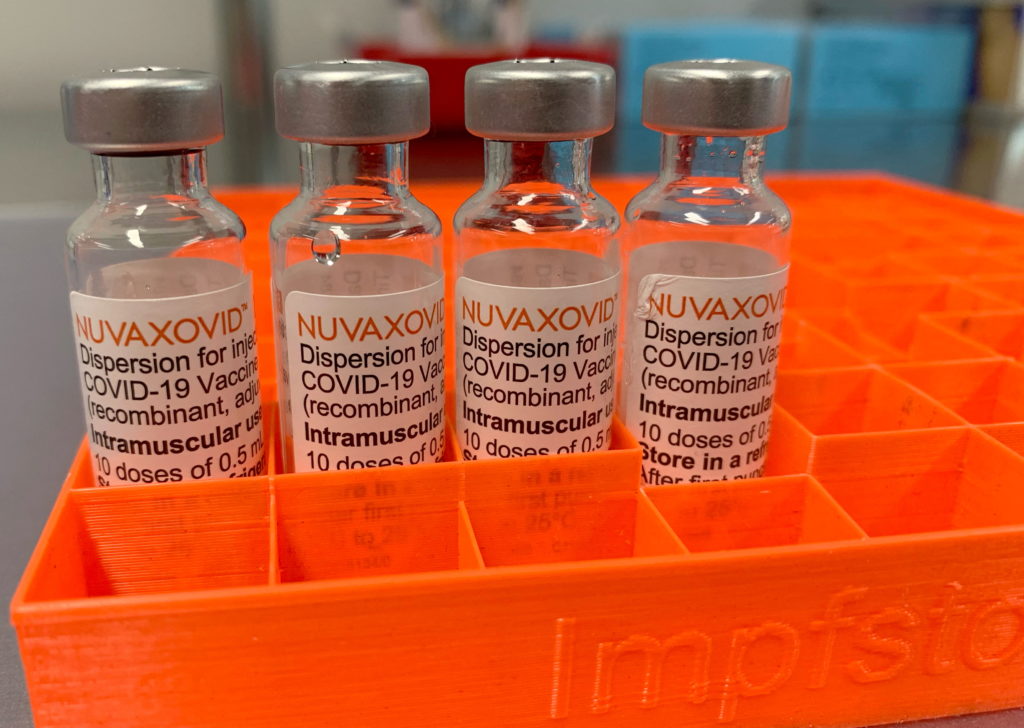
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, has been met with a mixture of relief and cautious optimism. While the authorization marks a significant step forward in the fight against the pandemic, offering a different vaccine technology to the public, it comes with stringent conditions that highlight ongoing concerns about safety and efficacy. This new protein subunit vaccine, unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, uses a more traditional approach, potentially appealing to those hesitant about newer technologies. However, the FDA's cautious approach underscores the need for continued monitoring and rigorous safety protocols.
A Different Approach to COVID-19 Vaccination
The approval of Nuvaxovid is a win for vaccine diversity. The vaccine utilizes a different technology than the dominant mRNA vaccines. It uses a protein subunit approach, presenting the body with harmless pieces of the virus to trigger an immune response. This methodology has been used in other established vaccines, such as the hepatitis B vaccine, potentially making it a more palatable option for vaccine-hesitant individuals who harbor concerns about mRNA technology. This approach could be particularly important for broadening vaccination efforts globally, especially in regions with limited access to ultra-cold storage required for mRNA vaccines.
Stringent Conditions and Ongoing Monitoring
Despite the approval, the FDA's authorization is accompanied by strict conditions. These conditions reflect the agency's commitment to ensuring the vaccine's safety and effectiveness. The FDA will continue to closely monitor the vaccine's safety profile through post-market surveillance, requiring Novavax to provide comprehensive data on adverse events and efficacy. This ongoing surveillance is crucial for detecting any unexpected side effects or changes in effectiveness over time. The stringent conditions imposed emphasize the regulatory body's dedication to public health and safety.
What the Strict Conditions Mean for the Public
The rigorous monitoring and reporting requirements mean that the FDA will be closely scrutinizing the performance and safety of Nuvaxovid. This is standard procedure for new vaccines, but the conditions highlight a cautious approach reflective of the evolving understanding of long-term effects of COVID-19 vaccination. For the public, this means that the FDA is prioritizing thorough data collection to ensure the safety and efficacy of the vaccine. It also signals a commitment to transparency and accountability in the vaccine approval process.
The Future of Nuvaxovid and COVID-19 Vaccination
The FDA's approval of Nuvaxovid, while cautiously optimistic, represents a potential game-changer in the COVID-19 vaccination landscape. The availability of a different vaccine technology provides a wider range of options for individuals, potentially addressing vaccine hesitancy and increasing vaccination rates globally. The ongoing monitoring and strict conditions demonstrate a commitment to both efficacy and safety, reassuring the public that the FDA is prioritizing public health. Further research and data analysis will be critical to fully understanding the long-term impact of Nuvaxovid and its role in broader COVID-19 prevention strategies. It is important to stay informed through reliable sources like the and your healthcare provider.
Keywords: FDA, Novavax, COVID-19 vaccine, Nuvaxovid, protein subunit vaccine, vaccine approval, vaccine safety, vaccine efficacy, mRNA vaccine, post-market surveillance, vaccine hesitancy, COVID-19 vaccination, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Greenlights Novavax COVID-19 Vaccine, But With Strict Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Trumps Intervention Russia And Ukraine To Engage In Immediate Truce Talks
May 21, 2025
Trumps Intervention Russia And Ukraine To Engage In Immediate Truce Talks
May 21, 2025 -
 2025 Pga Championship Schefflers Back Nine Charge Secures Triumph
May 21, 2025
2025 Pga Championship Schefflers Back Nine Charge Secures Triumph
May 21, 2025 -
 Solo Levelings Award Winning Success A Look At Future Nominations
May 21, 2025
Solo Levelings Award Winning Success A Look At Future Nominations
May 21, 2025 -
 Nba 2025 Championship Predictions Thunder Favored Knicks Strong Challengers
May 21, 2025
Nba 2025 Championship Predictions Thunder Favored Knicks Strong Challengers
May 21, 2025 -
 Brett Favre Documentary A Conversation With The Fall Of Favre Director
May 21, 2025
Brett Favre Documentary A Conversation With The Fall Of Favre Director
May 21, 2025
