FDA Grants Conditional Approval To Novavax COVID-19 Vaccine
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
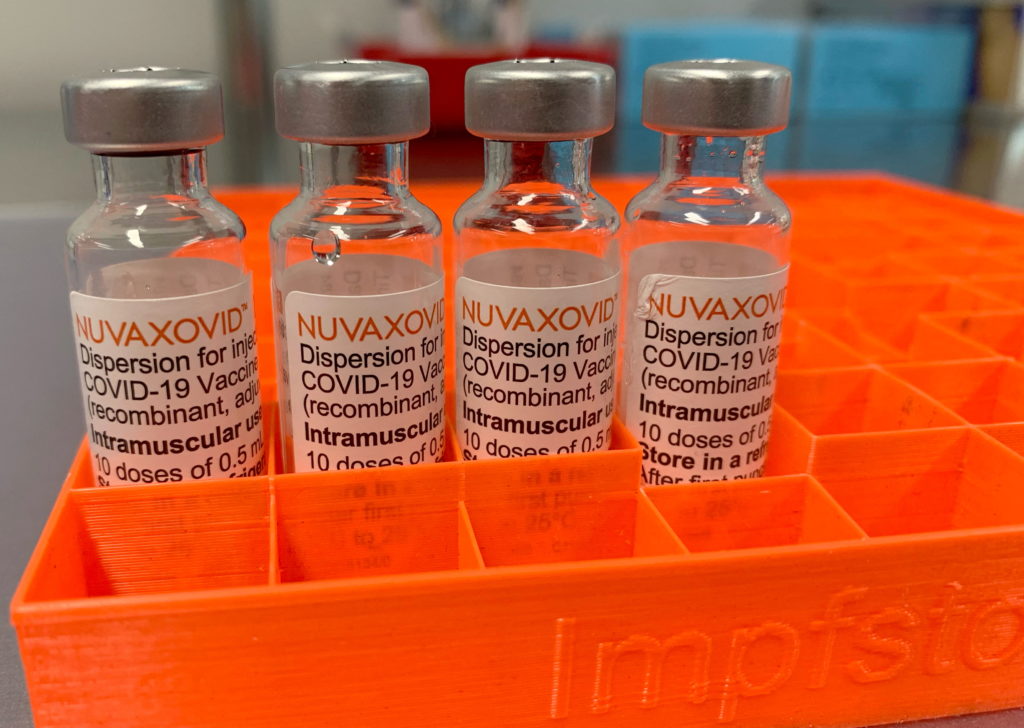
FDA Grants Conditional Approval to Novavax COVID-19 Vaccine: A New Option Arrives
The landscape of COVID-19 vaccination just expanded. On July 13, 2023, the U.S. Food and Drug Administration (FDA) granted conditional approval to Novavax's COVID-19 vaccine, Nuvaxovid (also known as Covovax), offering a new option for individuals seeking protection against the virus. This marks a significant milestone in the ongoing fight against the pandemic, providing an alternative for those who may have hesitated to receive mRNA vaccines.
This protein-subunit vaccine, unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, uses a different technology. This difference could be a game-changer for vaccine hesitancy. Many individuals expressed concerns about the novel mRNA technology used in the previously authorized vaccines. Novavax's approach employs a more traditional method, potentially addressing these concerns and broadening vaccine access.
How Does the Novavax Vaccine Work?
The Novavax vaccine uses a different mechanism than mRNA vaccines. Instead of delivering mRNA instructions to cells to produce the spike protein, it directly introduces the spike protein itself. This protein is produced in a lab and then formulated with an adjuvant – a substance that boosts the immune response. This process stimulates the body to produce antibodies against the COVID-19 virus, providing immunity.
This traditional approach might appeal to individuals hesitant about mRNA vaccines due to perceived risks or misconceptions. However, it's crucial to note that all authorized COVID-19 vaccines have undergone rigorous testing and have proven to be safe and highly effective at preventing severe illness, hospitalization, and death.
Who Can Receive the Novavax Vaccine?
Currently, the FDA's conditional approval is for individuals 18 years of age and older. Further trials are underway to assess its efficacy and safety in younger age groups. The vaccine is administered as a two-dose primary series, given three to eight weeks apart.
Addressing Vaccine Hesitancy: A Potential Turning Point?
The arrival of the Novavax vaccine offers a crucial opportunity to address vaccine hesitancy. The availability of a different vaccine technology may persuade those who previously declined vaccination due to concerns about mRNA vaccines to reconsider. This increased vaccine uptake could further contribute to herd immunity and ultimately help control the spread of COVID-19. Public health officials are hopeful that this new option will help bridge the gap and reach those who remain unvaccinated.
Beyond the Initial Approval: What's Next?
The FDA's conditional approval is a crucial step, but further monitoring and data analysis will continue. Post-market surveillance will be essential to track the vaccine's long-term safety and effectiveness. The CDC will also play a critical role in providing guidance to healthcare providers and the public regarding the vaccine's use and distribution.
Looking Ahead: The approval of the Novavax vaccine represents a significant development in the global fight against COVID-19. It provides a new avenue for boosting vaccination rates and protecting more people from the virus. Further research and ongoing surveillance will be key to ensuring its continued safety and effectiveness in the long term.
Disclaimer: This article is for informational purposes only and should not be considered medical advice. Consult with your healthcare provider to determine if the Novavax vaccine is right for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Grants Conditional Approval To Novavax COVID-19 Vaccine. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Perezs Head Scratcher The Ufl Storyline Dominating Week 8
May 21, 2025
Perezs Head Scratcher The Ufl Storyline Dominating Week 8
May 21, 2025 -
 2025 League Of Legends Hall Of Famer Revealed Will The Skin Be Too Expensive
May 21, 2025
2025 League Of Legends Hall Of Famer Revealed Will The Skin Be Too Expensive
May 21, 2025 -
 Watch Tyler Vaughns Spectacular Catch Leads Ufl Week 8 Highlights
May 21, 2025
Watch Tyler Vaughns Spectacular Catch Leads Ufl Week 8 Highlights
May 21, 2025 -
 Uzi Of League Of Legends Gifted An Electric G Wagon By Mercedes Benz
May 21, 2025
Uzi Of League Of Legends Gifted An Electric G Wagon By Mercedes Benz
May 21, 2025 -
 Suspended Referee Rooney Aims For Playoff Rematch
May 21, 2025
Suspended Referee Rooney Aims For Playoff Rematch
May 21, 2025
