FDA Grants Approval To Novavax COVID-19 Vaccine: Understanding The Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Grants Approval to Novavax COVID-19 Vaccine: Understanding the Conditions
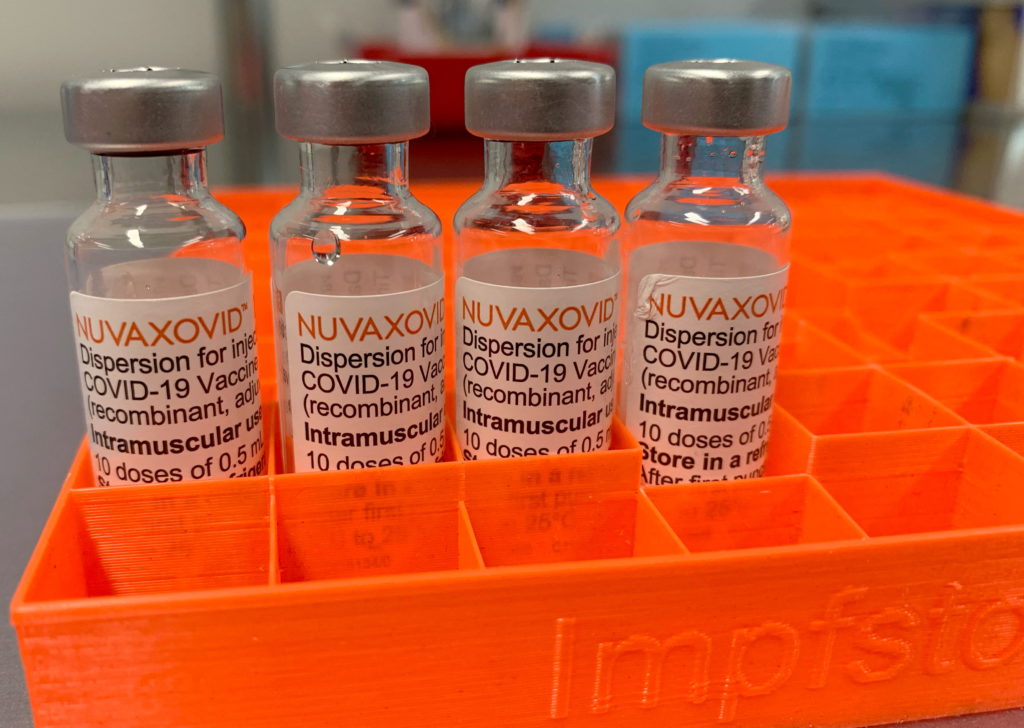
The FDA's full approval of the Novavax COVID-19 vaccine, Nuvaxovid, marks a significant milestone in the fight against the pandemic. This approval, however, comes with specific conditions, prompting important questions about its use and efficacy. Let's delve into the details.
A Long-Awaited Approval
After extensive trials and review, the Food and Drug Administration (FDA) granted full approval to Nuvaxovid for individuals 18 years of age and older. This contrasts with the previous Emergency Use Authorization (EUA) under which the vaccine was previously administered. Full approval signifies a more rigorous evaluation process, bolstering confidence in its safety and effectiveness. This approval is particularly relevant given the evolving COVID-19 landscape and the ongoing need for effective vaccination strategies.
Understanding the Conditions of Approval
While the approval is a positive development, it's crucial to understand the conditions placed upon Novavax by the FDA. These conditions are standard practice and ensure ongoing monitoring of the vaccine's safety and efficacy profile. This includes:
- Post-Market Surveillance: The FDA will continue to closely monitor the vaccine's safety and efficacy through robust post-market surveillance programs. This involves tracking adverse events and analyzing long-term data to assess its ongoing performance.
- Manufacturing Standards: Novavax must adhere to strict manufacturing standards and quality control measures to ensure consistent vaccine production. This is essential to maintaining the vaccine's potency and safety.
- Data Reporting: The company is obligated to submit regular reports to the FDA, including data on adverse events, manufacturing processes, and any significant updates regarding the vaccine's performance.
What Does This Mean for the Public?
For individuals hesitant to receive a COVID-19 vaccine due to concerns about mRNA technology, the approval of Novavax presents a protein-based alternative. The Novavax vaccine utilizes a different technology than the Pfizer-BioNTech and Moderna vaccines, offering a potentially preferable option for those with specific preferences or concerns. However, it's crucial to remember that all FDA-approved COVID-19 vaccines offer significant protection against severe illness, hospitalization, and death.
Addressing Common Concerns
Many individuals have questions about the Novavax vaccine's efficacy compared to other available options. While direct comparisons can be complex, the available data suggests that Nuvaxovid provides a comparable level of protection against severe COVID-19. It's important to consult with your healthcare provider to discuss which vaccine is the most suitable for your individual circumstances.
Looking Ahead
The full FDA approval of the Novavax COVID-19 vaccine strengthens the arsenal of tools available to combat the ongoing pandemic. The conditions of approval underscore the FDA's commitment to ensuring both safety and efficacy. This approval provides another important option for individuals seeking COVID-19 vaccination, ultimately contributing to improved public health. For the most up-to-date information, always refer to the CDC and FDA websites.
Call to Action: Talk to your doctor about which COVID-19 vaccine is right for you. Staying informed about vaccine updates and public health recommendations is crucial in protecting yourself and your community. Learn more at and .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Grants Approval To Novavax COVID-19 Vaccine: Understanding The Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 New Peaky Blinders Series Confirmed Expect The Unexpected
May 20, 2025
New Peaky Blinders Series Confirmed Expect The Unexpected
May 20, 2025 -
 Claim Your Rewards Helldivers 2 Masters Of Ceremony Warbond Drop May 15th
May 20, 2025
Claim Your Rewards Helldivers 2 Masters Of Ceremony Warbond Drop May 15th
May 20, 2025 -
 Bali Tourism New Guidelines Aim To Improve Tourist Behavior And Preserve The Island
May 20, 2025
Bali Tourism New Guidelines Aim To Improve Tourist Behavior And Preserve The Island
May 20, 2025 -
 Exclusive Interview The Making Of Netflixs Documentary Fall Of Favre
May 20, 2025
Exclusive Interview The Making Of Netflixs Documentary Fall Of Favre
May 20, 2025 -
 Major League Baseball Twins Correa And Buxton Out With Concussions Following In Game Collision
May 20, 2025
Major League Baseball Twins Correa And Buxton Out With Concussions Following In Game Collision
May 20, 2025
