FDA Grants Approval To Novavax COVID-19 Vaccine, Imposing Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Grants Approval to Novavax COVID-19 Vaccine, but with Usage Restrictions
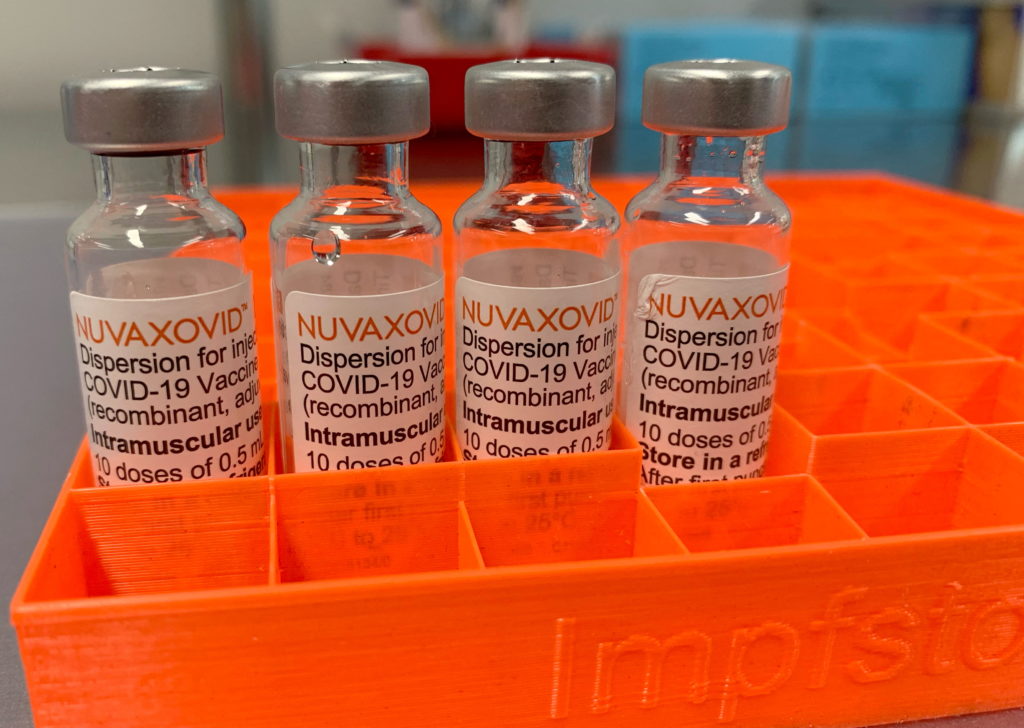
Novavax's COVID-19 vaccine, Nuvaxovid, finally receives full FDA approval, but with caveats. The long-awaited full approval from the Food and Drug Administration (FDA) marks a significant milestone for Novavax, but the agency's decision comes with important usage restrictions, impacting its potential reach and market share.
The FDA's approval, announced on [Insert Date of Announcement], grants Nuvaxovid full licensure for individuals 18 years of age and older. This contrasts with the previous Emergency Use Authorization (EUA), which allowed for broader, albeit temporary, use. However, the agency has imposed specific limitations, potentially impacting the vaccine's widespread adoption. Let's delve into the details.
What are the FDA's Usage Restrictions?
The FDA's approval of Nuvaxovid is not without conditions. The agency has placed specific restrictions based on its assessment of the available data. While the exact wording of these restrictions can be found on the FDA website [link to FDA website], key limitations appear to center around:
- Specific Age Group: Currently, the approval is limited to adults 18 years and older. Further studies are likely needed to assess its safety and efficacy in younger age groups.
- Limited Indications: The FDA's approval might be more narrowly defined than initially anticipated. This means the vaccine's recommended usage may be restricted to certain populations or situations.
- Potential Side Effects: Although generally well-tolerated, the FDA may have highlighted specific side effects necessitating close monitoring of patients post-vaccination. Understanding these potential side effects is crucial for healthcare providers and vaccine recipients alike.
This contrasts with other widely available COVID-19 vaccines, which have received broader authorizations. The restrictions imposed by the FDA raise questions about Nuvaxovid's market competitiveness.
Why the Restrictions? Understanding the FDA's Decision
The FDA's decision to grant full approval alongside specific usage restrictions reflects a cautious, data-driven approach. While the vaccine demonstrated efficacy and safety in clinical trials, the agency likely considered several factors before granting full approval. These may include:
- Comparative Efficacy Data: A thorough comparison of Nuvaxovid's efficacy against other available COVID-19 vaccines likely played a significant role in the FDA's decision-making process.
- Long-Term Safety Data: The FDA may have required more extensive long-term safety data before granting unrestricted approval. Gathering such data takes time and contributes to the overall regulatory review process.
- Manufacturing Process: The FDA’s review likely included a stringent assessment of Novavax's manufacturing processes and quality control measures to ensure consistent vaccine production.
Understanding these factors provides crucial context to the FDA’s decision. The restrictions aren't necessarily indicative of significant safety concerns, but rather a reflection of the rigorous standards applied during the approval process.
What Does this Mean for the Future of Nuvaxovid?
The FDA's approval, while conditional, is still a significant step for Novavax. The full approval may help bolster public confidence and facilitate wider uptake, particularly amongst individuals hesitant to receive mRNA vaccines. However, the imposed restrictions will likely impact the vaccine's market penetration. The company will likely need to conduct further studies to expand the approved age range and indications. The future success of Nuvaxovid hinges on navigating these regulatory hurdles and demonstrating its continued value in the evolving COVID-19 landscape.
Call to Action: Stay informed about the latest developments concerning COVID-19 vaccines by consulting your healthcare provider and reliable sources like the CDC and FDA websites. Remember to consult your doctor before making any decisions regarding vaccination.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Grants Approval To Novavax COVID-19 Vaccine, Imposing Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Heated Wnba Debut Caitlin Clarks Triple Double And Confrontation With Angel Reese
May 20, 2025
Heated Wnba Debut Caitlin Clarks Triple Double And Confrontation With Angel Reese
May 20, 2025 -
 Market Rally Continues Six Day Win Streak For S And P 500 Amidst Moodys Negative Outlook
May 20, 2025
Market Rally Continues Six Day Win Streak For S And P 500 Amidst Moodys Negative Outlook
May 20, 2025 -
 Yankees Vs Soto Uncomfortable Memories In The Bronx
May 20, 2025
Yankees Vs Soto Uncomfortable Memories In The Bronx
May 20, 2025 -
 Exclusive Lionel Messi Speaks On Inter Miamis Current Challenges
May 20, 2025
Exclusive Lionel Messi Speaks On Inter Miamis Current Challenges
May 20, 2025 -
 Juego De Voces 2025 Anuncian Invitados Especiales Para La Gran Final
May 20, 2025
Juego De Voces 2025 Anuncian Invitados Especiales Para La Gran Final
May 20, 2025
