FDA Approves Novavax COVID-19 Vaccine: Use Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approves Novavax COVID-19 Vaccine: Use Restrictions Explained
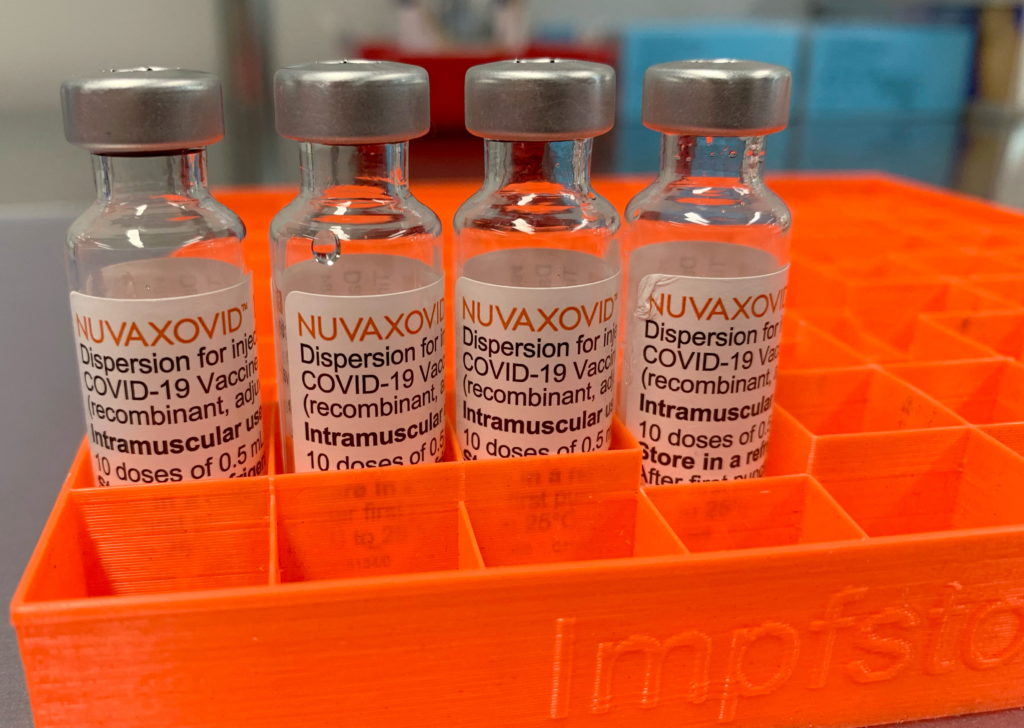
The FDA has granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, offering a new option for vaccination in the United States. However, understanding the use restrictions is crucial before scheduling your shot.
The arrival of Novavax's COVID-19 vaccine, Nuvaxovid, marks a significant development in the fight against the pandemic. This protein subunit vaccine offers a different mechanism of action compared to the mRNA vaccines already widely available, such as Pfizer-BioNTech and Moderna. This alternative could be particularly appealing to individuals hesitant about mRNA technology. But the FDA's approval comes with specific use restrictions that are vital to understand.
Understanding Novavax's Nuvaxovid: A Protein Subunit Vaccine
Unlike mRNA vaccines that use messenger RNA to instruct cells to produce a viral protein, Novavax's vaccine utilizes a different approach. It uses a lab-made version of the spike protein found on the surface of the SARS-CoV-2 virus. This protein is combined with an adjuvant, a substance that enhances the immune response. This method has been used in other established vaccines for years, potentially increasing public trust and acceptance.
FDA-Approved Use: Who Can Get the Novavax Vaccine?
The FDA's EUA for Nuvaxovid specifies its use for individuals 18 years of age and older. This is a key restriction – children and adolescents cannot currently receive this vaccine. Further clinical trials are needed to determine its safety and efficacy in younger populations. The agency emphasized the importance of following the recommended dosage and administration guidelines provided by the manufacturer.
Key Use Restrictions and Considerations:
- Age Limitation: Currently, only adults aged 18 and older are eligible.
- Two-Dose Regimen: The vaccine requires two doses administered several weeks apart. Precise timing should be followed as indicated on the vaccine packaging and by your healthcare provider.
- Allergies: Individuals with known severe allergic reactions to any component of the vaccine, including the adjuvant, should not receive it. It's crucial to disclose all allergies to your healthcare provider before vaccination.
- Pre-existing Conditions: While generally safe, individuals with pre-existing conditions should consult their doctor to assess any potential risks or interactions. This is standard practice for any vaccination.
- Post-Vaccination Monitoring: As with any vaccine, it is essential to monitor for potential side effects after receiving each dose. Common side effects are typically mild and short-lived, but serious reactions are rare.
Where Can I Get the Novavax Vaccine?
The availability of the Novavax vaccine will vary depending on your location. Check with your doctor, local pharmacies, and healthcare providers to determine where you can access it. The provides updated information on vaccine distribution and availability.
The Future of Novavax and COVID-19 Vaccination
The FDA's approval of the Novavax vaccine represents a significant step forward. Offering a different vaccine technology provides an alternative for those who may have hesitated to receive mRNA vaccines. Further research and potential approval for younger age groups will enhance the options available in the fight against COVID-19. The long-term impact of this vaccine and its role in future booster campaigns remains to be seen, but its arrival is a welcome addition to the vaccination arsenal.
Disclaimer: This article provides general information and should not be considered medical advice. Consult your healthcare provider for any concerns related to COVID-19 vaccination.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Use Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Wnba Responds To Fan Misconduct Clark Reese Incident Prompts Inquiry
May 20, 2025
Wnba Responds To Fan Misconduct Clark Reese Incident Prompts Inquiry
May 20, 2025 -
 Nascar All Star Race At North Wilkesboro Recap Of The Top Moments And Live Race Updates
May 20, 2025
Nascar All Star Race At North Wilkesboro Recap Of The Top Moments And Live Race Updates
May 20, 2025 -
 Win Big Best Mlb Walk Off Wager Predictions For White Sox Red Sox And Other Games
May 20, 2025
Win Big Best Mlb Walk Off Wager Predictions For White Sox Red Sox And Other Games
May 20, 2025 -
 La Intensa Semana De Carreras Del Real Madrid Un Analisis Detallado
May 20, 2025
La Intensa Semana De Carreras Del Real Madrid Un Analisis Detallado
May 20, 2025 -
 Better World Fund Honors Kevin Spacey Analyzing The Actors Return To The Spotlight
May 20, 2025
Better World Fund Honors Kevin Spacey Analyzing The Actors Return To The Spotlight
May 20, 2025
