FDA Approves Novavax COVID-19 Vaccine: Use Restricted
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
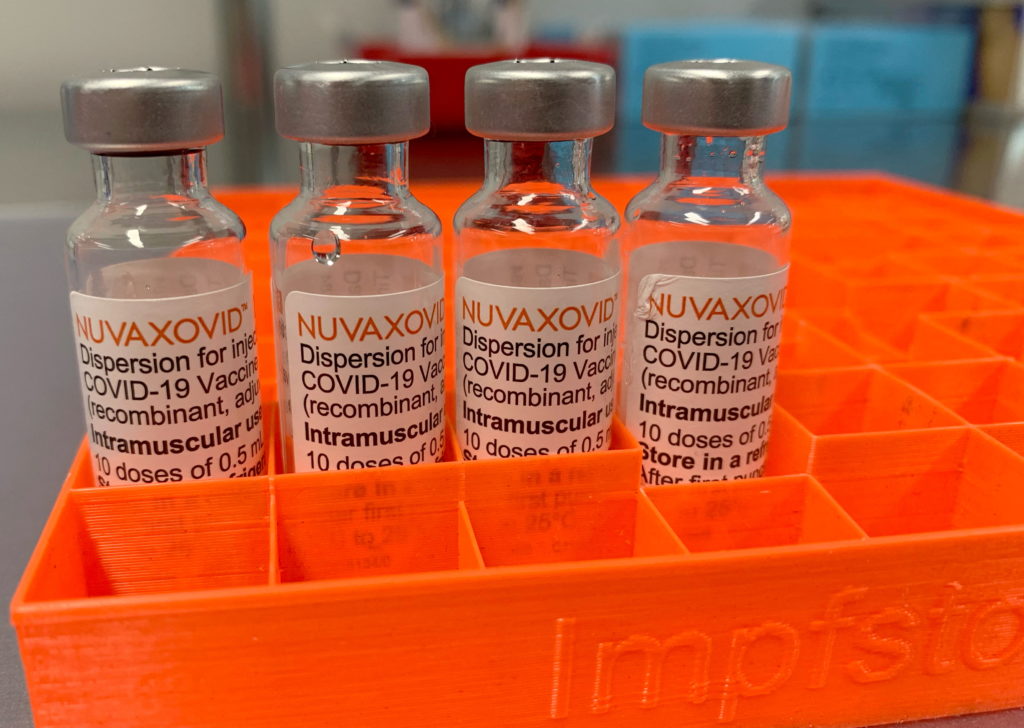
FDA Approves Novavax COVID-19 Vaccine: Use Restricted to Adults Only
The FDA has granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, but with a significant caveat: its use is currently restricted to adults 18 years of age and older. This announcement, while welcomed by some as offering an alternative vaccine technology, also raises questions about the timeline for broader authorization and the vaccine's overall impact on the pandemic.
The approval marks a significant milestone for Novavax, whose protein-subunit vaccine utilizes a different technology than the mRNA vaccines from Pfizer-BioNTech and Moderna, and the viral vector vaccine from Johnson & Johnson. This difference could be crucial for individuals hesitant to receive mRNA vaccines due to concerns about the novel technology. The protein-subunit approach uses a more traditional method, potentially appealing to a wider segment of the population.
Understanding Nuvaxovid: A Protein-Subunit Vaccine
Unlike mRNA vaccines that instruct the body's cells to produce viral proteins, Nuvaxovid uses lab-made pieces of the virus (spike protein) to trigger an immune response. This approach has been used in other vaccines for decades, potentially increasing public confidence. The FDA's review process included a comprehensive analysis of safety and efficacy data, culminating in this EUA.
Why the Age Restriction?
The FDA's decision to restrict the vaccine's use to adults is based on the available data. While clinical trials demonstrated efficacy and safety in adults, sufficient data on the vaccine's effectiveness and safety in younger age groups is still pending. Further trials are ongoing to evaluate the vaccine's performance in adolescents and children. This phased approach is standard practice in vaccine rollout and prioritizes safety.
What Does This Mean for the COVID-19 Vaccination Campaign?
The addition of Nuvaxovid to the arsenal of available COVID-19 vaccines is a positive step, potentially expanding access to those who may have been hesitant to receive other vaccines. However, the restricted use limits its immediate impact. The availability of the vaccine will depend on manufacturing capacity and distribution strategies.
Key takeaways:
- Alternative Technology: Nuvaxovid offers a protein-subunit approach, different from mRNA vaccines.
- Adult-Only Authorization: Currently approved only for individuals 18 years and older.
- Ongoing Trials: Further trials are needed to determine safety and efficacy in younger age groups.
- Potential Impact: May increase vaccination rates among those hesitant about mRNA vaccines.
Looking Ahead: Future Prospects for Nuvaxovid
The FDA's approval is a crucial step, but more data is needed before wider authorization can be considered. Novavax is actively pursuing further clinical trials to expand the age range for Nuvaxovid. The success of these trials will determine the vaccine's ultimate role in the ongoing fight against COVID-19. The company also plans to submit data to regulatory bodies globally, paving the way for broader international availability.
The approval of Novavax's vaccine provides a valuable option, particularly for adults who may prefer a protein-subunit approach. However, the age restriction underscores the ongoing need for rigorous testing and data analysis in vaccine development. For more information on COVID-19 vaccines and vaccination recommendations, consult the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Use Restricted. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Nine Days Later Angel Citys Christen Press Leaves Hospital Post Collapse
May 20, 2025
Nine Days Later Angel Citys Christen Press Leaves Hospital Post Collapse
May 20, 2025 -
 Rahms Pga Collapse Disappointment But Undeterred
May 20, 2025
Rahms Pga Collapse Disappointment But Undeterred
May 20, 2025 -
 Putins Calculated Move Demonstrating Independence From Trump
May 20, 2025
Putins Calculated Move Demonstrating Independence From Trump
May 20, 2025 -
 Super Regional Showdown Libertys Upset Victory Over Texas A And M
May 20, 2025
Super Regional Showdown Libertys Upset Victory Over Texas A And M
May 20, 2025 -
 New Legislation Proposed Addressing The Pet Cremation Industry Scandal
May 20, 2025
New Legislation Proposed Addressing The Pet Cremation Industry Scandal
May 20, 2025
