FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Understanding the Restrictions
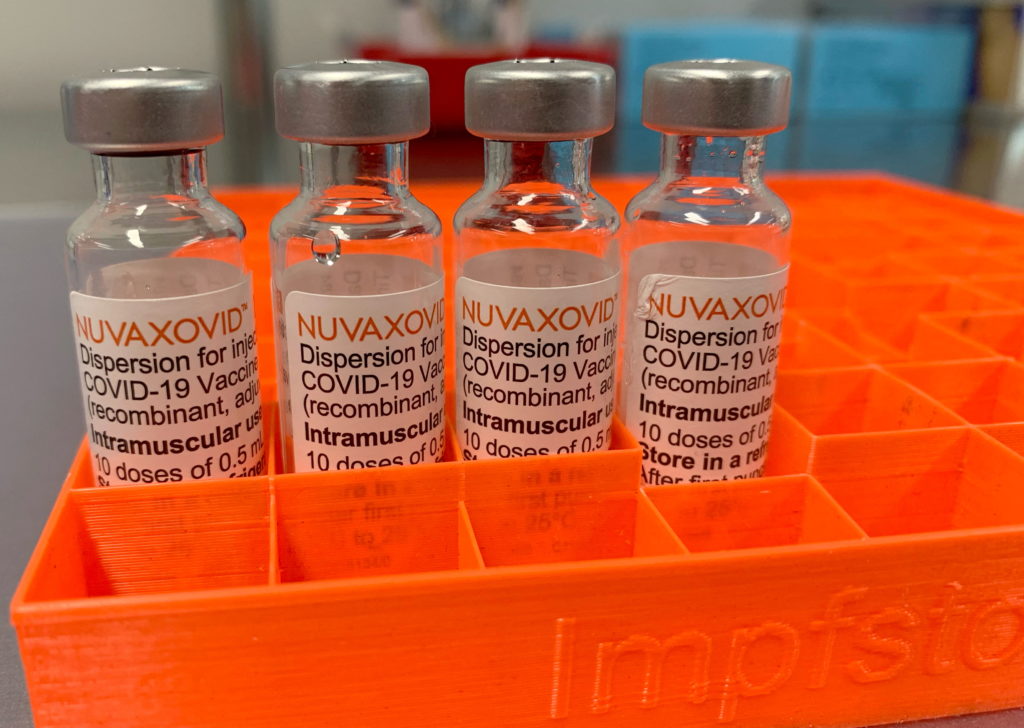
The Novavax COVID-19 vaccine, Nuvaxovid, finally received full FDA approval in July 2023, marking a significant milestone in the fight against the pandemic. However, this approval comes with certain restrictions, prompting questions and concerns among healthcare professionals and the public. This article will delve into the details of the FDA's approval, outlining the limitations and providing clarity on its use.
What Does Full FDA Approval Mean?
Full FDA approval, also known as a Biologics License Application (BLA) approval, signifies a rigorous review process exceeding the Emergency Use Authorization (EUA) pathway. The FDA scrutinized extensive clinical trial data demonstrating the vaccine's safety and effectiveness against COVID-19. This approval differs significantly from the EUA process, offering increased confidence in the vaccine's long-term safety profile. For consumers, this means a greater level of assurance regarding the vaccine's quality and efficacy.
Restrictions on Novavax COVID-19 Vaccine Use:
While full approval is a positive step, the FDA's authorization for Nuvaxovid includes specific restrictions. These limitations primarily revolve around:
- Age Restrictions: The FDA approval is currently limited to individuals 18 years of age and older. Further clinical trials are needed to establish the safety and efficacy of the vaccine in younger age groups.
- Dosage: The approved dosage and administration method are explicitly defined. Any deviation from these guidelines requires specific medical justification.
- Specific Indications: The approval focuses on preventing COVID-19 caused by the original strain of the SARS-CoV-2 virus. While it offers some protection against variants, its efficacy against newer variants might be reduced. This warrants continued monitoring and potential adjustments to the vaccine formulation in the future. [Link to CDC information on COVID-19 variants].
- Adverse Events Monitoring: Post-market surveillance is crucial. The FDA mandates continued monitoring of adverse events associated with the vaccine's usage to ensure ongoing safety and efficacy.
Comparison to Other COVID-19 Vaccines:
Several other COVID-19 vaccines are already widely available with full FDA approval. Nuvaxovid's approval adds another tool to the arsenal against the virus, particularly offering an alternative for individuals who may have hesitations about mRNA vaccines. However, it's important to remember that all vaccines carry potential side effects, and the severity and frequency of side effects vary between individuals and vaccines. [Link to CDC information comparing COVID-19 vaccines].
The Importance of Vaccination:
Despite the restrictions, the FDA approval of the Novavax vaccine underscores the importance of continued vaccination efforts against COVID-19. Vaccination remains a cornerstone of public health strategies in mitigating the pandemic’s impact. Understanding the specific limitations of each vaccine allows for informed decision-making and helps to build public trust in vaccination programs.
Moving Forward:
The FDA's approval of the Novavax vaccine represents progress, but ongoing research and monitoring are essential. Future studies will likely focus on expanding the age range for its use, assessing its effectiveness against emerging variants, and further solidifying its long-term safety profile. The ongoing battle against COVID-19 necessitates a multifaceted approach, and the availability of multiple vaccines, each with its own characteristics and limitations, contributes to a more effective and comprehensive strategy.
Call to Action: Consult your healthcare provider to determine the most suitable COVID-19 vaccine for your individual needs and circumstances. Staying informed about vaccine updates and recommendations is crucial in protecting yourself and your community.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 U S Treasury Yield Decline One 2025 Fed Rate Cut Expected
May 20, 2025
U S Treasury Yield Decline One 2025 Fed Rate Cut Expected
May 20, 2025 -
 Against All Odds Thunder Oust Nuggets Clinch Spot In Western Conference Finals
May 20, 2025
Against All Odds Thunder Oust Nuggets Clinch Spot In Western Conference Finals
May 20, 2025 -
 A J Perez Reveals Intimidation From Brett Favres Camp Following Untold Documentary
May 20, 2025
A J Perez Reveals Intimidation From Brett Favres Camp Following Untold Documentary
May 20, 2025 -
 Galactus Promo Art Unveiled Marvels Fantastic Four First Steps
May 20, 2025
Galactus Promo Art Unveiled Marvels Fantastic Four First Steps
May 20, 2025 -
 Pga Championship Jon Rahms Final Round Collapse And Positive Outlook
May 20, 2025
Pga Championship Jon Rahms Final Round Collapse And Positive Outlook
May 20, 2025
