FDA Approval For Novavax COVID-19 Vaccine: Understanding The Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
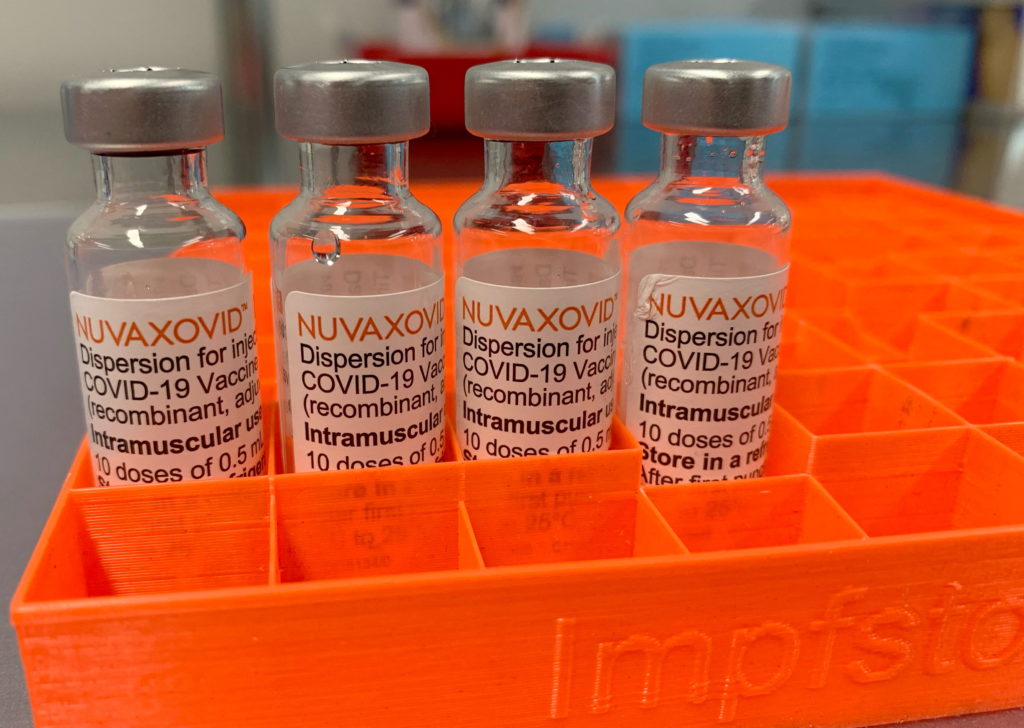
FDA Approval for Novavax COVID-19 Vaccine: Understanding the Conditions
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, marks a significant step in the fight against the pandemic. However, it's crucial to understand the conditions surrounding this approval to ensure informed decision-making. This isn't just another vaccine on the market; its unique technology and approval pathway warrant careful consideration.
What Makes Novavax Different?
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax uses a more traditional protein subunit technology. This involves producing harmless pieces of the virus (spike proteins) to trigger an immune response. This approach has been used in other vaccines for years, potentially appealing to those hesitant about newer technologies. This difference is key to understanding the nuances of its FDA approval.
The FDA Approval: A Conditional Green Light
The FDA granted full approval to Nuvaxovid for individuals 18 years and older, but it's important to note the term "full approval" in this context. While it signifies a higher level of scrutiny than Emergency Use Authorization (EUA), it's still contingent on ongoing monitoring and data collection. The FDA's approval is conditional upon Novavax meeting certain post-market requirements, ensuring long-term safety and efficacy.
Key Conditions of Approval:
- Continued Monitoring of Adverse Events: Post-market surveillance will closely track the vaccine's safety profile, looking for any rare or unexpected side effects. This is standard practice for all newly approved vaccines.
- Manufacturing Process Oversight: The FDA will continue to oversee Novavax's manufacturing processes to ensure consistent quality and purity of the vaccine. This is vital to maintaining vaccine efficacy and safety.
- Data Submission Requirements: Novavax is required to submit ongoing data to the FDA, including updated safety and efficacy information from real-world use. This ensures the agency has the most up-to-date information on the vaccine's performance.
Who Should Consider Novavax?
The Novavax vaccine presents an alternative for individuals who may have preferred a more traditional vaccine technology. However, it's crucial to consult with your healthcare provider to determine which COVID-19 vaccine is best suited for your individual health needs and circumstances. Factors such as pre-existing conditions and potential allergies should be carefully considered.
Addressing Vaccine Hesitancy:
The approval of Novavax could help address vaccine hesitancy among some individuals who were wary of mRNA technology. The familiar protein subunit approach might increase confidence and uptake. However, it's essential to emphasize that all approved COVID-19 vaccines offer a high degree of protection against severe illness, hospitalization, and death.
Looking Ahead:
The FDA's approval of the Novavax vaccine represents a valuable addition to the arsenal of COVID-19 vaccines available. Ongoing monitoring and data collection are essential to ensure its long-term safety and efficacy. This underscores the importance of continued public health efforts, including vaccination and booster shots, to mitigate the ongoing impact of the pandemic. Further research and development will continue to refine our understanding of COVID-19 and improve preventative measures. Stay informed and consult your healthcare provider for the most up-to-date information.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Understanding The Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Okc Thunder Rout Denver Ending Nuggets Playoff Run In Commanding Fashion
May 20, 2025
Okc Thunder Rout Denver Ending Nuggets Playoff Run In Commanding Fashion
May 20, 2025 -
 Toledo Mud Hens Rout Opponent With 20 Run Offensive Outburst Liranzo Continues Hot Streak
May 20, 2025
Toledo Mud Hens Rout Opponent With 20 Run Offensive Outburst Liranzo Continues Hot Streak
May 20, 2025 -
 Post Pectra Upgrade Ethereum Sees 200 Million Influx In Investment
May 20, 2025
Post Pectra Upgrade Ethereum Sees 200 Million Influx In Investment
May 20, 2025 -
 Robert Shwartzmans Indy 500 Odds Soar After Securing Pole In 2025
May 20, 2025
Robert Shwartzmans Indy 500 Odds Soar After Securing Pole In 2025
May 20, 2025 -
 Trae Young Praises Thunder Fans Mocks Knicks A Look At The Nba Rivalry
May 20, 2025
Trae Young Praises Thunder Fans Mocks Knicks A Look At The Nba Rivalry
May 20, 2025
