FDA Approval For Novavax COVID-19 Vaccine: Specific Use Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Specific Use Restrictions Explained
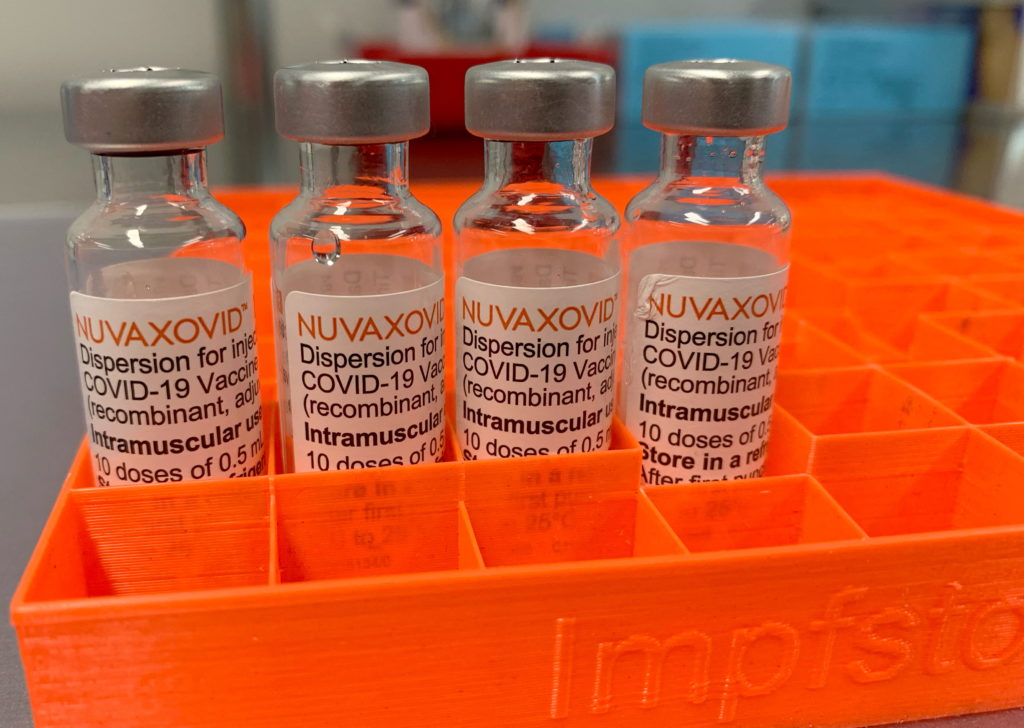
The Novavax COVID-19 vaccine, Nuvaxovid, has received FDA approval, marking a significant development in the fight against the pandemic. However, this approval comes with specific use restrictions that are crucial for healthcare providers and the public to understand. This article will break down these restrictions and explain their implications.
What is Nuvaxovid?
Nuvaxovid is a protein-subunit COVID-19 vaccine, differing from mRNA vaccines like Pfizer-BioNTech and Moderna. It uses a more traditional approach, employing lab-grown pieces of the virus's spike protein to trigger an immune response. This technology has been used in other vaccines for decades, potentially addressing some hesitancy surrounding mRNA technology. The vaccine is administered in two doses, given three to eight weeks apart.
FDA Approval and its Significance
The FDA's approval of Nuvaxovid signifies a rigorous review process and confirmation of its safety and efficacy. While previously available under Emergency Use Authorization (EUA), the full approval provides increased confidence in its use. This approval offers another option for individuals seeking COVID-19 vaccination, particularly those who may have been hesitant about mRNA vaccines.
Understanding the Use Restrictions:
While the approval is a positive step, the FDA has imposed specific use restrictions:
- Age Restriction: Currently, Nuvaxovid is approved only for individuals 18 years of age and older. Further studies are needed to determine its safety and efficacy in younger populations.
- Dosage: The vaccine is administered as a two-dose primary series, with a specific interval between doses. Deviation from the recommended dosage regimen should be avoided.
- Pre-existing Conditions: Individuals with a history of severe allergic reactions to any component of the vaccine should not receive it. Consult a healthcare provider if you have concerns about pre-existing conditions.
- Adverse Events: While generally well-tolerated, Nuvaxovid, like other vaccines, can cause mild side effects such as pain at the injection site, fatigue, headache, muscle aches, and nausea. Serious adverse events are rare. Individuals experiencing severe reactions should seek immediate medical attention.
- Efficacy against Variants: The vaccine's efficacy against emerging COVID-19 variants is constantly monitored and evaluated. The FDA's approval reflects its efficacy against the variants prevalent at the time of review. Further studies are ongoing to assess its performance against future variants.
Who Should Consider Novavax?
Nuvaxovid offers a valuable option for individuals who:
- Prefer a protein-subunit vaccine over mRNA vaccines.
- Have concerns about mRNA vaccine technology.
- Meet the age and other eligibility criteria outlined by the FDA.
Important Considerations:
It's crucial to consult with your healthcare provider to determine if Nuvaxovid is the right choice for you. They can assess your individual health status and provide personalized recommendations based on your medical history and risk factors. Remember to discuss any concerns or questions you have regarding the vaccine before receiving it.
Staying Informed:
For the latest information on COVID-19 vaccines, including Nuvaxovid, refer to the official websites of the FDA () and the CDC (). Staying informed is crucial for making informed decisions about your health and well-being.
Disclaimer: This article provides general information and does not constitute medical advice. Consult with a healthcare professional for personalized recommendations.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Specific Use Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Balis Plea International Collaboration For Responsible Tourism
May 20, 2025
Balis Plea International Collaboration For Responsible Tourism
May 20, 2025 -
 Jon Rahms Pga Collapse Disappointment And Determination
May 20, 2025
Jon Rahms Pga Collapse Disappointment And Determination
May 20, 2025 -
 Nuggets Success Coach David Adelmans Leadership Praised By Players
May 20, 2025
Nuggets Success Coach David Adelmans Leadership Praised By Players
May 20, 2025 -
 Wall Street Defies Moody S S And P 500 Extends Winning Streak Market Analysis
May 20, 2025
Wall Street Defies Moody S S And P 500 Extends Winning Streak Market Analysis
May 20, 2025 -
 Indy 500 Practice A Look At The Weekends Numerous Accidents
May 20, 2025
Indy 500 Practice A Look At The Weekends Numerous Accidents
May 20, 2025
