FDA Approval For Novavax COVID-19 Vaccine: Specific Usage Restrictions Announced
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Specific Usage Restrictions Announced
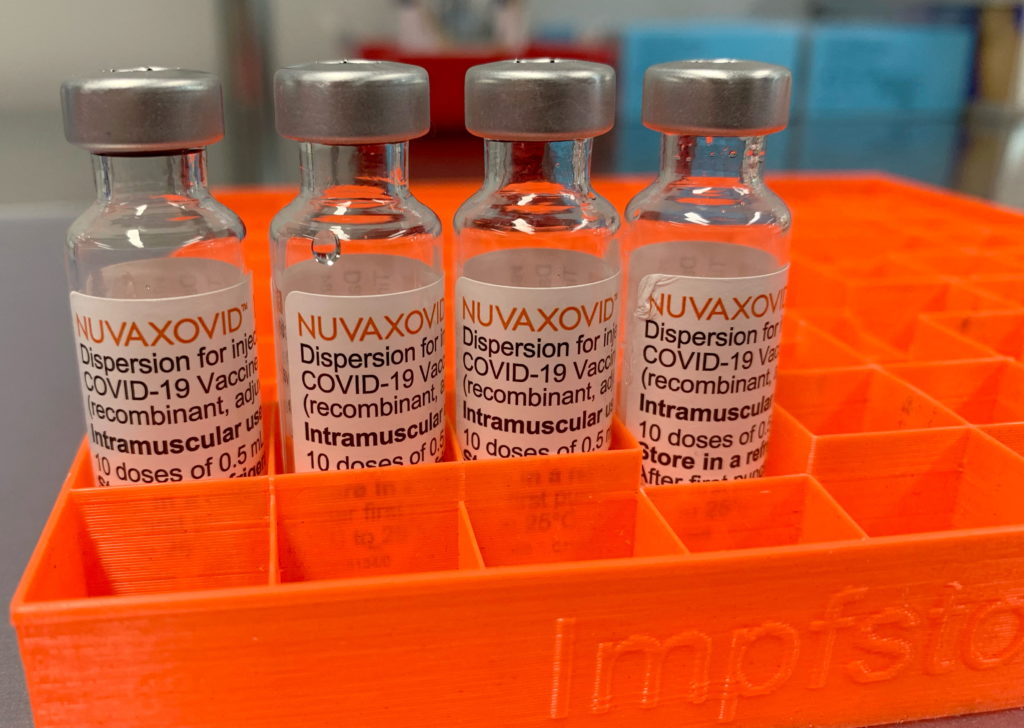
The FDA has granted full approval to Novavax's COVID-19 vaccine, Nuvaxovid, but with specific usage restrictions. This landmark decision, while welcomed by many, also introduces nuances that healthcare providers and the public need to understand. The approval marks a significant step in the fight against the pandemic, offering an alternative vaccine option for those who may have hesitated to receive mRNA vaccines. However, understanding the limitations is crucial for safe and effective deployment.
What does full FDA approval mean?
Full approval, unlike emergency use authorization (EUA), signifies a more rigorous review process by the FDA. This involves a comprehensive assessment of the vaccine's safety and efficacy data, including long-term studies. It signifies a higher level of confidence in the vaccine's performance and safety profile. This approval should increase public confidence and potentially lead to wider adoption.
Nuvaxovid: A Different Approach
Unlike the Pfizer-BioNTech and Moderna vaccines, which use mRNA technology, Novavax's Nuvaxovid is a protein subunit vaccine. This means it uses a piece of the virus's protein to trigger an immune response. This technology has been used in other vaccines for years, potentially making it a more familiar and appealing option for some individuals hesitant about mRNA vaccines. This difference in technology is key to understanding the specific restrictions.
Specific Usage Restrictions Announced by the FDA:
The FDA's approval of Nuvaxovid comes with specific caveats:
- Age Restrictions: The vaccine is currently approved for individuals 18 years of age and older. Further studies are needed to determine its safety and efficacy in younger populations.
- Dosage: The vaccine is administered as a two-dose primary series, given three to eight weeks apart. Booster doses may be recommended in the future, pending further data.
- Known Allergies: Individuals with a known history of severe allergic reactions to any component of the vaccine should not receive it. As with all vaccines, monitoring for allergic reactions post-vaccination is crucial.
- Pre-existing Conditions: Individuals with certain pre-existing conditions may need to discuss the vaccine's suitability with their healthcare provider.
Addressing Concerns and Misinformation:
The FDA's approval process aims to address public concerns about vaccine safety and efficacy. This full approval, coupled with the transparently stated restrictions, should help dispel misinformation and encourage informed decision-making. It's vital to consult reliable sources like the CDC and FDA websites for accurate information.
What does this mean for the future of COVID-19 vaccination?
The approval of Nuvaxovid provides a valuable alternative in the fight against COVID-19. Offering a different vaccine technology expands access for those who might have previously been hesitant due to concerns about mRNA vaccines. However, the specific usage restrictions highlight the ongoing need for comprehensive research and individualized risk assessments. Continued monitoring of the vaccine's long-term effects and ongoing research into broader approvals, particularly for younger age groups, will be crucial.
Call to Action:
Consult your healthcare provider to determine if the Novavax COVID-19 vaccine is the right choice for you. Stay informed about updates from reliable sources like the and the . Vaccination remains a vital tool in protecting yourself and your community from COVID-19.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Specific Usage Restrictions Announced. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 2024 Western Conference Finals Thunder Overcome Nuggets End Seven Year Drought
May 20, 2025
2024 Western Conference Finals Thunder Overcome Nuggets End Seven Year Drought
May 20, 2025 -
 Jon Rahm Reflects On Near Miss At Pga Championship A Look Ahead
May 20, 2025
Jon Rahm Reflects On Near Miss At Pga Championship A Look Ahead
May 20, 2025 -
 Concussion Concerns Correa And Buxton Hit The Il Following Twins Collision
May 20, 2025
Concussion Concerns Correa And Buxton Hit The Il Following Twins Collision
May 20, 2025 -
 5 B Invested The Rise Of Bitcoin Etfs And The Implications For The Market
May 20, 2025
5 B Invested The Rise Of Bitcoin Etfs And The Implications For The Market
May 20, 2025 -
 Wwi Epic Starring Daniel Craig Cillian Murphy And Tom Hardy Where To Watch
May 20, 2025
Wwi Epic Starring Daniel Craig Cillian Murphy And Tom Hardy Where To Watch
May 20, 2025
