FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Stipulations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
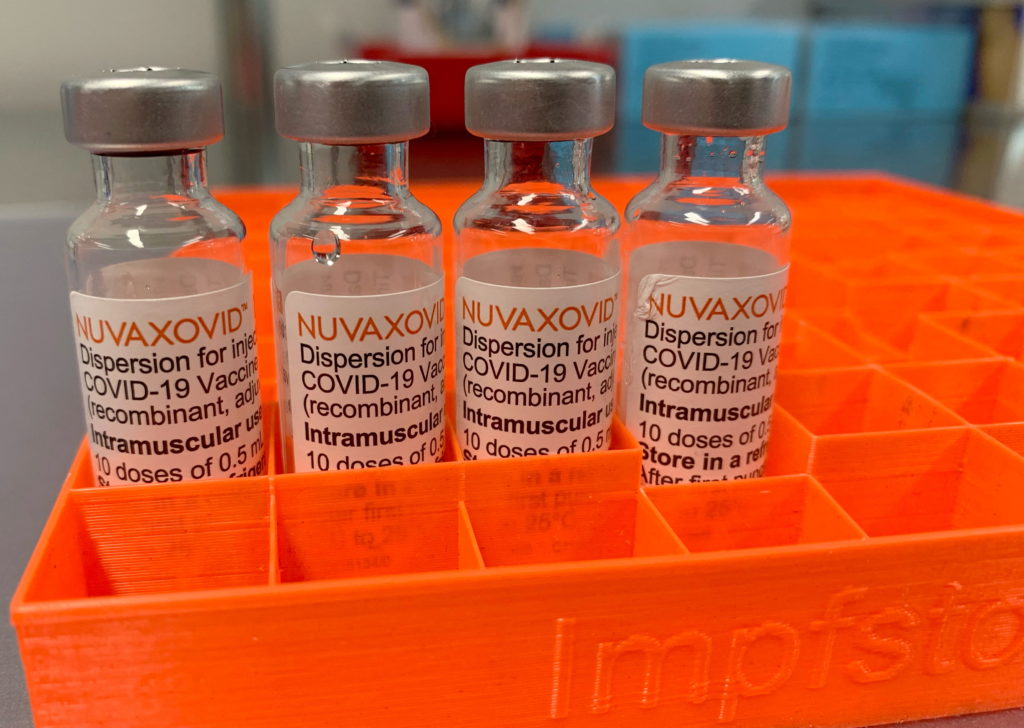
FDA Approval for Novavax COVID-19 Vaccine Comes with Unusual Stipulations
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, has been met with a mix of relief and raised eyebrows. While hailed as a welcome addition to the arsenal against the virus, the approval comes with some unusual stipulations that have sparked debate among health experts and the public. This marks a significant milestone for Novavax, but the conditions attached raise questions about the future of vaccine development and distribution.
A Protein Subunit Vaccine: A Different Approach
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Nuvaxovid utilizes a protein subunit technology. This approach uses harmless pieces of the virus to trigger an immune response, a method considered more traditional and potentially less prone to certain side effects. This difference in technology is a key factor in understanding the FDA's unique approach to its approval. Many see this as a potential boon for vaccine hesitant individuals who might be more receptive to a protein-based vaccine.
The Unusual Stipulations: Limited Authorization and Manufacturing Concerns
The FDA's approval is not without its caveats. The agency has granted only a limited approval, focusing on individuals 18 years of age and older. This differs from the broader age ranges covered by other authorized vaccines. Furthermore, the approval is contingent upon Novavax meeting specific manufacturing quality control requirements. This implies concerns about the consistency and reliability of the vaccine's production process.
Specifically, the FDA cites ongoing issues with the vaccine's manufacturing process, requiring Novavax to meet rigorous standards before wider distribution can occur. This unusual condition reflects a more cautious approach by the FDA compared to other vaccine approvals. The agency is prioritizing safety and efficacy above rapid deployment in this instance.
What does this mean for the public?
The limited approval means Nuvaxovid’s availability might be initially constrained, potentially affecting its impact on overall vaccination rates. The focus on manufacturing quality is a positive sign, demonstrating the FDA's commitment to safety, even if it means a slower rollout. For individuals hesitant about mRNA vaccines, the protein subunit technology might offer a more appealing option, but its limited availability could be a significant drawback.
Looking Ahead: Impact on Vaccine Landscape and Future Approvals
The FDA's decision highlights the complexities of vaccine development and approval. The stipulations placed on Novavax's vaccine underscore the agency's commitment to rigorous standards, even if it means a less straightforward approval process. This case will likely set a precedent for future vaccine approvals, potentially influencing the scrutiny applied to other innovative vaccine technologies. The coming months will be crucial in observing how Novavax addresses the FDA's concerns and how the vaccine is ultimately integrated into existing vaccination strategies.
Frequently Asked Questions (FAQs)
- Is the Novavax vaccine safe? The FDA has approved the vaccine, indicating it meets their safety and efficacy standards. However, as with all vaccines, potential side effects exist.
- When will the Novavax vaccine be widely available? This depends on Novavax meeting the FDA's manufacturing requirements. Wider availability is expected, but the timeframe remains uncertain.
- Is the Novavax vaccine effective against current COVID-19 variants? While the initial data showed efficacy against earlier variants, ongoing research is needed to determine its effectiveness against newer variants. [Link to CDC website on COVID-19 variants]
This development underscores the ongoing evolution of the COVID-19 pandemic and the constant adaptation of our strategies to combat it. The Novavax approval, despite its conditions, offers another layer of protection and reinforces the importance of vaccination in our collective efforts.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Stipulations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Panthers Clinching Game 7 Win Sends Maple Leafs Home
May 21, 2025
Panthers Clinching Game 7 Win Sends Maple Leafs Home
May 21, 2025 -
 Nfl 2024 Which Backup Quarterbacks Could Unexpectedly Shine
May 21, 2025
Nfl 2024 Which Backup Quarterbacks Could Unexpectedly Shine
May 21, 2025 -
 Get Master Of Ceremony Warbonds In Helldivers 2 On May 15th
May 21, 2025
Get Master Of Ceremony Warbonds In Helldivers 2 On May 15th
May 21, 2025 -
 Solo Levelings Award Winning Success A Look At Its Rising Popularity
May 21, 2025
Solo Levelings Award Winning Success A Look At Its Rising Popularity
May 21, 2025 -
 Kyle Schwarber Launches 300th Home Run A Mammoth 466 Footer
May 21, 2025
Kyle Schwarber Launches 300th Home Run A Mammoth 466 Footer
May 21, 2025
