FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine Comes With Unusual Restrictions
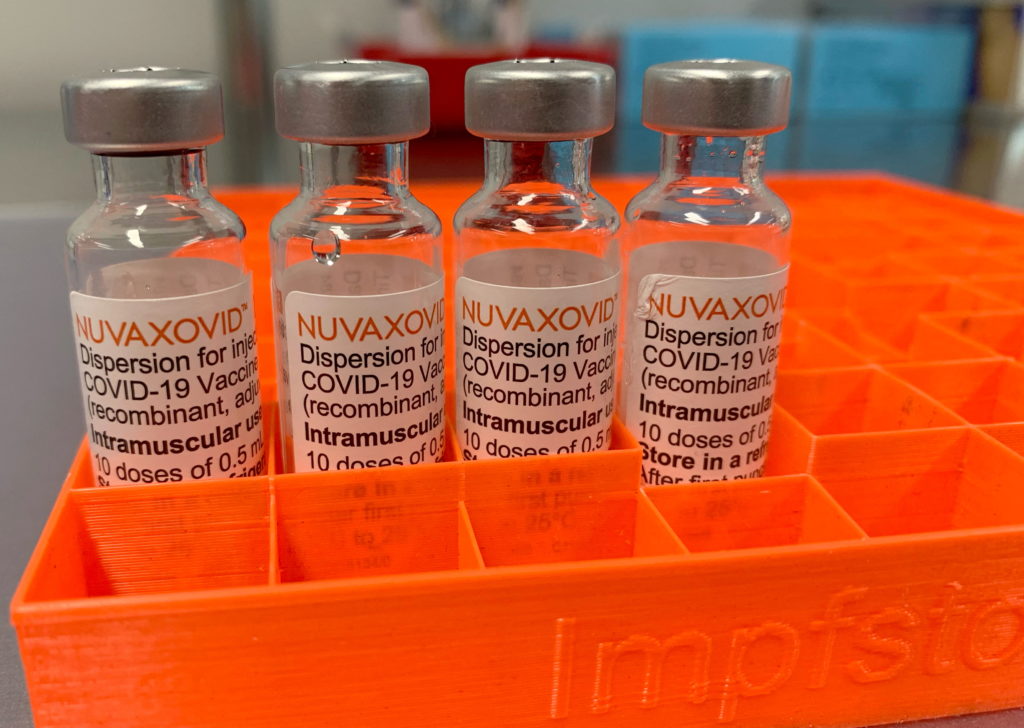
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, finally arrived, ending a long wait for a protein-subunit vaccine option. However, this approval comes with a significant caveat: unusual and arguably restrictive limitations on its use. This raises questions about the vaccine's future role in the ongoing fight against the pandemic. While many celebrated the arrival of a different vaccine technology, the FDA's conditions are prompting discussions about access and efficacy.
Why the Unusual Restrictions?
The FDA's approval isn't a blanket green light. The agency has granted approval only for individuals 18 years of age and older. This is in contrast to other authorized COVID-19 vaccines, some of which are approved for use in younger age groups. The reasons behind this age restriction haven't been fully clarified by the FDA, fueling speculation and concern among public health experts.
Furthermore, the agency's approval is contingent upon ongoing safety monitoring and data collection. This heightened surveillance suggests potential concerns regarding the vaccine's long-term safety profile that need further investigation. This is an atypical level of scrutiny compared to other previously approved vaccines.
What are the implications of these restrictions?
These restrictions could significantly limit the vaccine's impact. The age limitation excludes a considerable portion of the population, particularly those who may have been hesitant to receive mRNA vaccines. The added surveillance requirement might also lead to delays in vaccine rollout and distribution.
- Reduced Access: The age restriction alone could severely hamper the number of people who might choose Novavax, narrowing its potential effectiveness in broader population immunity.
- Supply Chain Concerns: The added monitoring might further complicate the already complex vaccine supply chain, potentially causing delays in getting the vaccine to those who need it most.
- Public Perception: The unusual restrictions could negatively impact public perception, leading to increased hesitancy and contributing to lower uptake rates.
Comparing Novavax to other COVID-19 Vaccines
Novavax utilizes a different technology than the widely used mRNA vaccines (Pfizer-BioNTech and Moderna) and viral vector vaccines (Johnson & Johnson). It's a protein-subunit vaccine, which uses a harmless piece of the virus to trigger an immune response. This technology has been used for decades in other vaccines, potentially appealing to individuals wary of newer mRNA technology. However, the FDA's restrictions highlight that even established technologies require rigorous scrutiny and ongoing monitoring. You can learn more about the different vaccine types on the .
Looking Ahead:
The FDA's conditional approval of the Novavax vaccine presents a complex scenario. While offering an alternative vaccine technology, the associated restrictions raise significant questions about accessibility and widespread adoption. The long-term impact on vaccination rates and herd immunity remains to be seen. Ongoing monitoring and further clarification from the FDA are crucial to fully understand the implications of this decision and address any potential concerns. The future of Novavax's role in the fight against COVID-19 will depend heavily on how these restrictions are addressed and whether the vaccine can overcome the hurdles presented. This situation underscores the ongoing need for transparent communication and robust scientific investigation in vaccine development and deployment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Leaked Funko Pop First Steps Hints At A Significant Addition To The Fantastic Four
May 20, 2025
Leaked Funko Pop First Steps Hints At A Significant Addition To The Fantastic Four
May 20, 2025 -
 Denver Nuggets Adelman Receives Strong Player Endorsement Positive Coach Reviews
May 20, 2025
Denver Nuggets Adelman Receives Strong Player Endorsement Positive Coach Reviews
May 20, 2025 -
 Novavax Covid 19 Vaccine Fda Approval And Usage Restrictions Explained
May 20, 2025
Novavax Covid 19 Vaccine Fda Approval And Usage Restrictions Explained
May 20, 2025 -
 Indy 500 2025 Betting Preview Shwartzmans Pole Changes The Game
May 20, 2025
Indy 500 2025 Betting Preview Shwartzmans Pole Changes The Game
May 20, 2025 -
 Jamie Lee Curtis Gives An Exclusive Update On Her Friendship With Lindsay Lohan After Their Hit Film
May 20, 2025
Jamie Lee Curtis Gives An Exclusive Update On Her Friendship With Lindsay Lohan After Their Hit Film
May 20, 2025
