FDA Approval For Novavax COVID-19 Vaccine Comes With Strict Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
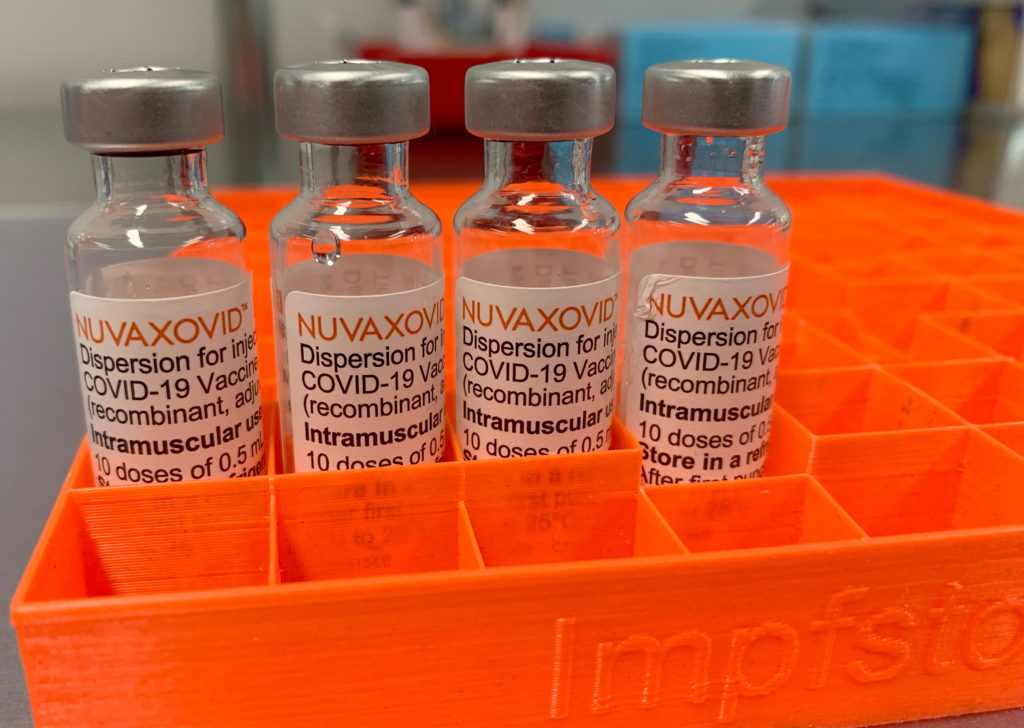
FDA Approval for Novavax COVID-19 Vaccine Comes with Strict Conditions
The FDA has finally granted full approval to Novavax's COVID-19 vaccine, Nuvaxovid, but the approval comes with a significant caveat: strict conditions tied to manufacturing and ongoing monitoring. This landmark decision, announced on [Insert Date of Announcement], marks a significant step for Novavax, but also highlights the rigorous standards the FDA applies even after years into the pandemic. The approval is a victory for the company, but the conditions underscore the ongoing scrutiny surrounding COVID-19 vaccines.
What the FDA Approval Means
Full approval from the FDA signifies that the agency has completed a comprehensive review of Nuvaxovid's safety and efficacy data, finding it to meet their high standards. This differs from the previous Emergency Use Authorization (EUA), providing increased confidence for individuals hesitant to receive a vaccine under EUA. This approval also opens doors for broader distribution and potential inclusion in government mandates and insurance coverage. However, the road to full market access isn't without obstacles.
Strict Conditions Imposed by the FDA
The FDA's approval is not unconditional. The agency has imposed stringent conditions on Novavax, primarily focused on:
-
Manufacturing Oversight: The FDA will maintain rigorous oversight of Novavax's manufacturing processes. This includes ongoing inspections and stringent quality control checks to ensure consistent product quality and prevent any deviations from approved standards. This level of scrutiny reflects concerns raised earlier regarding manufacturing inconsistencies reported by other vaccine manufacturers.
-
Post-Market Surveillance: Novavax is obligated to conduct extensive post-market surveillance to continuously monitor the vaccine's safety and effectiveness. This involves collecting and analyzing data on adverse events and efficacy in real-world settings, allowing for swift action if any issues arise. This data will be crucial in ensuring long-term safety and efficacy profiles.
-
Compliance with GMP standards: The approval hinges on Novavax's strict adherence to Good Manufacturing Practices (GMP) guidelines. Any failure to comply with these guidelines could result in the revocation of the approval. This emphasizes the critical role of manufacturing integrity in vaccine production.
Implications for Vaccine Hesitancy
The FDA's decision, despite the conditions, could potentially sway some vaccine-hesitant individuals. The full approval might offer a degree of reassurance, particularly those who preferred a protein-subunit vaccine like Nuvaxovid over mRNA vaccines. However, misinformation continues to be a significant challenge. Clear and accurate communication about the vaccine's benefits and the rationale behind the FDA's conditions will be critical to address concerns and maximize uptake.
The Future of Nuvaxovid
The full approval represents a significant milestone for Novavax. However, the accompanying conditions underscore the challenges involved in bringing a new vaccine to market, even during a global health crisis. The ongoing monitoring and stringent manufacturing requirements highlight the FDA's unwavering commitment to ensuring vaccine safety and efficacy. The success of Nuvaxovid in the long term hinges on Novavax's ability to consistently meet these conditions.
Call to Action: For further information on COVID-19 vaccines and the FDA approval process, visit the FDA website [link to FDA website] and the CDC website [link to CDC website]. Consult with your healthcare provider to discuss whether Nuvaxovid is the right vaccine for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine Comes With Strict Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Trump Calls For Immediate Russia Ukraine Peace Negotiations
May 20, 2025
Trump Calls For Immediate Russia Ukraine Peace Negotiations
May 20, 2025 -
 Nuggets Coach David Adelman Praise And Support From The Team
May 20, 2025
Nuggets Coach David Adelman Praise And Support From The Team
May 20, 2025 -
 Knicks Vs Thunder Trae Youngs Controversial Fan Remarks
May 20, 2025
Knicks Vs Thunder Trae Youngs Controversial Fan Remarks
May 20, 2025 -
 Imposing Galactus New Promo Art For Marvels Fantastic Four
May 20, 2025
Imposing Galactus New Promo Art For Marvels Fantastic Four
May 20, 2025 -
 Early 2025 Nba Title Odds Thunder And Knicks Vie For Championship
May 20, 2025
Early 2025 Nba Title Odds Thunder And Knicks Vie For Championship
May 20, 2025
