Conditional FDA Approval: What You Need To Know About The Novavax COVID-19 Vaccine
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: What You Need to Know About the Novavax COVID-19 Vaccine
The COVID-19 pandemic dramatically altered our lives, and vaccines emerged as a crucial tool in combating the virus. While several vaccines received widespread use, the arrival of the Novavax COVID-19 vaccine marked a significant development, offering a different approach to immunization. This article delves into the details surrounding its conditional FDA approval and what you need to know before considering this vaccine.
Understanding Conditional FDA Approval
Before diving into the specifics of the Novavax vaccine, it's crucial to understand what "conditional approval" means. Unlike a full FDA approval, which comes after extensive long-term studies, conditional approval allows for the quicker release of a vaccine deemed safe and effective based on available data. This accelerated pathway is often utilized during public health emergencies like the COVID-19 pandemic to rapidly make life-saving treatments accessible. However, the FDA continues to monitor the vaccine's safety and efficacy even after conditional approval, requiring ongoing data submission from the manufacturer. This ensures that any potential long-term side effects or issues are promptly identified and addressed.
Novavax: A Different Approach to COVID-19 Vaccination
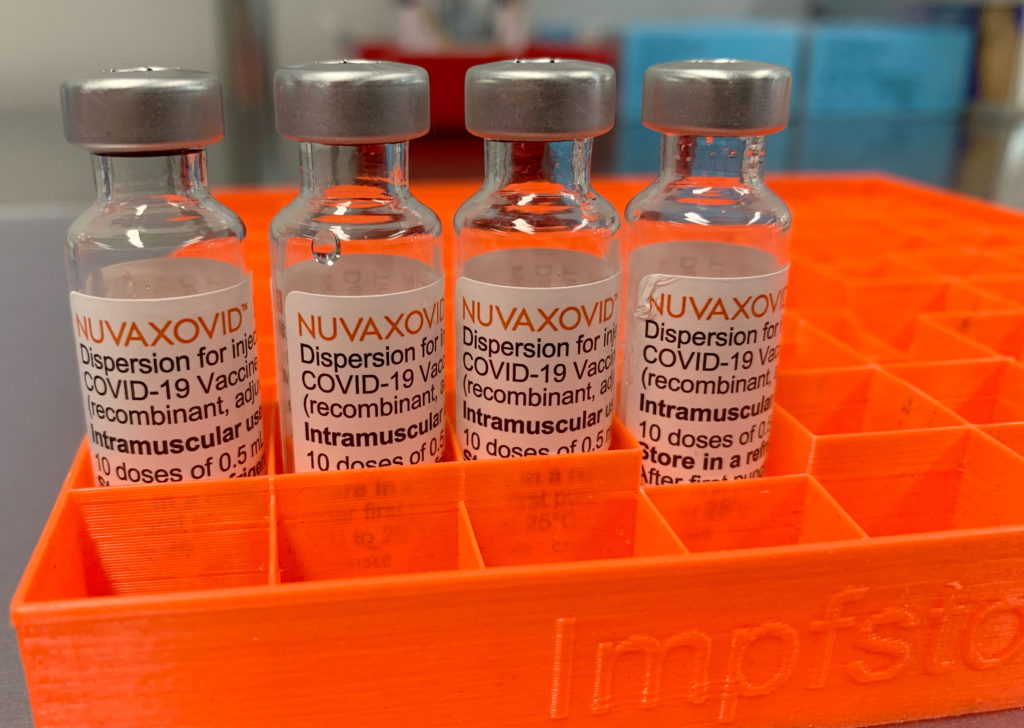
The Novavax COVID-19 vaccine, marketed as Nuvaxovid, uses a different technology compared to mRNA vaccines like Pfizer-BioNTech and Moderna. Instead of mRNA, it utilizes a protein subunit technology. This involves using harmless pieces of the virus's spike protein to trigger an immune response. This approach has been used in other vaccines for years, offering a potentially familiar and reassuring alternative for those hesitant about mRNA technology. This difference in technology is a key factor for many individuals considering their vaccination options.
Efficacy and Safety of the Novavax Vaccine
Clinical trials demonstrated the Novavax vaccine's effectiveness against COVID-19, showing a strong immune response and protection against severe illness. The FDA's conditional approval was based on rigorous evaluation of these clinical trial data, demonstrating the vaccine's safety and efficacy profile. Like all vaccines, side effects are possible, though most are mild and temporary. Common side effects reported include pain at the injection site, fatigue, headache, muscle aches, and joint pain. Serious side effects are rare. You can find comprehensive information on reported side effects on the .
Who Should Consider the Novavax Vaccine?
The Novavax vaccine is authorized for individuals 18 years of age and older. It might be a particularly suitable option for individuals who:
- Have concerns about mRNA vaccine technology.
- Prefer a protein subunit vaccine.
- Have experienced adverse reactions to other COVID-19 vaccines.
However, it's crucial to consult with your healthcare provider to discuss your individual medical history and determine the most appropriate vaccine for you.
Staying Informed and Making Informed Decisions
The availability of the Novavax vaccine adds another layer to the ongoing fight against COVID-19. Understanding its conditional approval, its technology, and its safety profile empowers you to make informed decisions about your vaccination strategy. Remember to always consult with your doctor or other qualified healthcare professional to discuss your specific circumstances and receive personalized advice. Staying informed through reputable sources like the and websites is also essential. The ongoing monitoring of the vaccine's long-term effects assures the public of continuous evaluation and adjustments as needed, ensuring both safety and efficacy remain paramount.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: What You Need To Know About The Novavax COVID-19 Vaccine. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Lionel Messi Interview Honest Assessment And Hope For Inter Miamis Success
May 20, 2025
Lionel Messi Interview Honest Assessment And Hope For Inter Miamis Success
May 20, 2025 -
 Free Mlb Home Run Predictions May 17th Marte Wood And Top Hr Prop Bets
May 20, 2025
Free Mlb Home Run Predictions May 17th Marte Wood And Top Hr Prop Bets
May 20, 2025 -
 Rahms Challenge Falls Short Schefflers Pga Championship Triumph
May 20, 2025
Rahms Challenge Falls Short Schefflers Pga Championship Triumph
May 20, 2025 -
 Amidst Rising Military Tensions Taiwans President Advocates For Peace Through Dialogue With China
May 20, 2025
Amidst Rising Military Tensions Taiwans President Advocates For Peace Through Dialogue With China
May 20, 2025 -
 Post Pectra Upgrade 200 M Flood Of Investment Into Ethereum Funds
May 20, 2025
Post Pectra Upgrade 200 M Flood Of Investment Into Ethereum Funds
May 20, 2025
