Conditional FDA Approval: Understanding The Novavax COVID-19 Vaccine's Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Understanding the Novavax COVID-19 Vaccine's Limitations
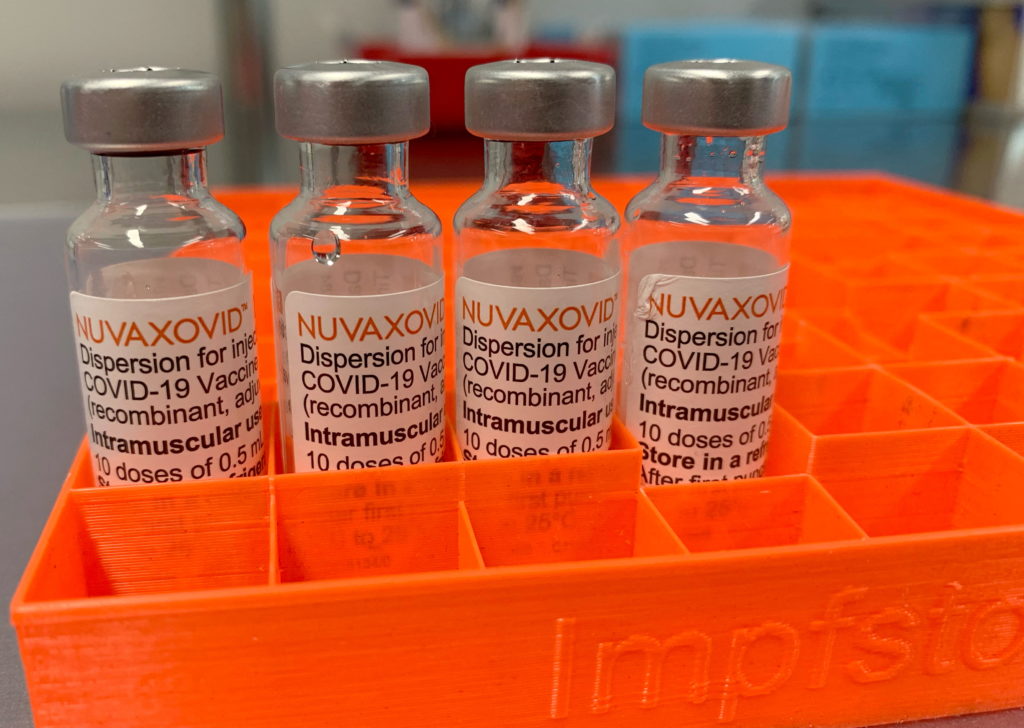
The Novavax COVID-19 vaccine, Nuvaxovid, finally received conditional FDA approval in July 2023, offering a protein-subunit option to those hesitant about mRNA vaccines. However, this approval comes with caveats. Understanding the limitations of Novavax's vaccine is crucial for informed decision-making, especially given the evolving COVID-19 landscape. This article delves into the specifics of the conditional approval and the key factors to consider.
What is Conditional FDA Approval?
Unlike a full FDA approval, conditional approval signifies that the agency has deemed the vaccine's benefits outweigh its risks based on available data, but further studies are required to confirm its long-term efficacy and safety. This often means that more data needs to be collected over time to solidify the approval. This approach is common for novel vaccines or treatments, allowing quicker access for the public while continuing the rigorous scientific evaluation.
Novavax Vaccine: A Different Approach
Unlike the Pfizer-BioNTech and Moderna vaccines which use mRNA technology, Novavax employs a protein-subunit approach. This involves using harmless pieces of the virus's protein to trigger an immune response. Many believe this traditional approach might alleviate some of the concerns surrounding mRNA technology, although it's important to note that both approaches have proven effective.
Limitations of the Novavax COVID-19 Vaccine:
Several limitations are associated with the Novavax vaccine's conditional approval:
-
Lower Efficacy Compared to mRNA Vaccines: Clinical trials showed slightly lower efficacy against infection compared to the mRNA vaccines, particularly against newer variants. While still effective at preventing severe illness and hospitalization, this difference is a key consideration. [Link to a relevant CDC or FDA study on comparative efficacy]
-
Limited Data on Long-Term Efficacy and Safety: The conditional approval highlights a need for more extensive long-term data. While short-term safety profiles appear favorable, ongoing monitoring is essential to fully understand the long-term effects.
-
Logistical Challenges: The Novavax vaccine requires a more complex cold chain storage system compared to some mRNA vaccines, potentially impacting its distribution and accessibility in certain regions. [Link to relevant article discussing logistical challenges of vaccine distribution]
-
Variant-Specific Efficacy: The effectiveness of the Novavax vaccine against emerging variants remains an area of ongoing research. While it has shown efficacy against the original strain and some variants, its performance against future mutations needs further evaluation. [Link to research on vaccine effectiveness against specific variants]
Who Should Consider the Novavax Vaccine?
The Novavax vaccine might be a suitable option for individuals who:
- Prefer a protein-subunit vaccine over mRNA technology.
- Have experienced adverse reactions to other COVID-19 vaccines. (Always consult with a healthcare provider before making vaccination decisions.)
However, it's crucial to weigh the potential benefits against its limitations, and to discuss this with a healthcare professional to make an informed decision.
Conclusion:
The conditional FDA approval of the Novavax COVID-19 vaccine represents a significant step in providing diverse vaccination options. However, it's vital to understand the limitations associated with this vaccine, including its slightly lower efficacy compared to mRNA vaccines and the need for further long-term safety and efficacy data. Informed decision-making regarding COVID-19 vaccination requires careful consideration of individual risk factors, personal preferences, and available scientific evidence. Consult your doctor to determine the best COVID-19 vaccine for your individual needs.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Understanding The Novavax COVID-19 Vaccine's Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Pga Leaderboard Schefflers 65 Sets Up Thrilling Final Round Showdown
May 20, 2025
Pga Leaderboard Schefflers 65 Sets Up Thrilling Final Round Showdown
May 20, 2025 -
 Minnesota Twins Shatter Record With 13 Game Winning Streak
May 20, 2025
Minnesota Twins Shatter Record With 13 Game Winning Streak
May 20, 2025 -
 Helldivers 2 Master Of Ceremony Warbond Drop Starts May 15th
May 20, 2025
Helldivers 2 Master Of Ceremony Warbond Drop Starts May 15th
May 20, 2025 -
 Nhls Scheifele Tragedy And Triumph In Jets Defeat
May 20, 2025
Nhls Scheifele Tragedy And Triumph In Jets Defeat
May 20, 2025 -
 Bali Cracks Down On Bad Tourist Behavior New Guidelines Unveiled
May 20, 2025
Bali Cracks Down On Bad Tourist Behavior New Guidelines Unveiled
May 20, 2025
