Conditional FDA Approval: Novavax COVID-19 Vaccine's Restricted Use
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine's Restricted Use
The FDA's conditional approval of the Novavax COVID-19 vaccine marks a significant development in the ongoing battle against the pandemic, but with important caveats. While offering an alternative for those hesitant about mRNA vaccines, its restricted use highlights the complexities of vaccine rollout and the evolving landscape of COVID-19 immunity.
Understanding the Conditional Approval
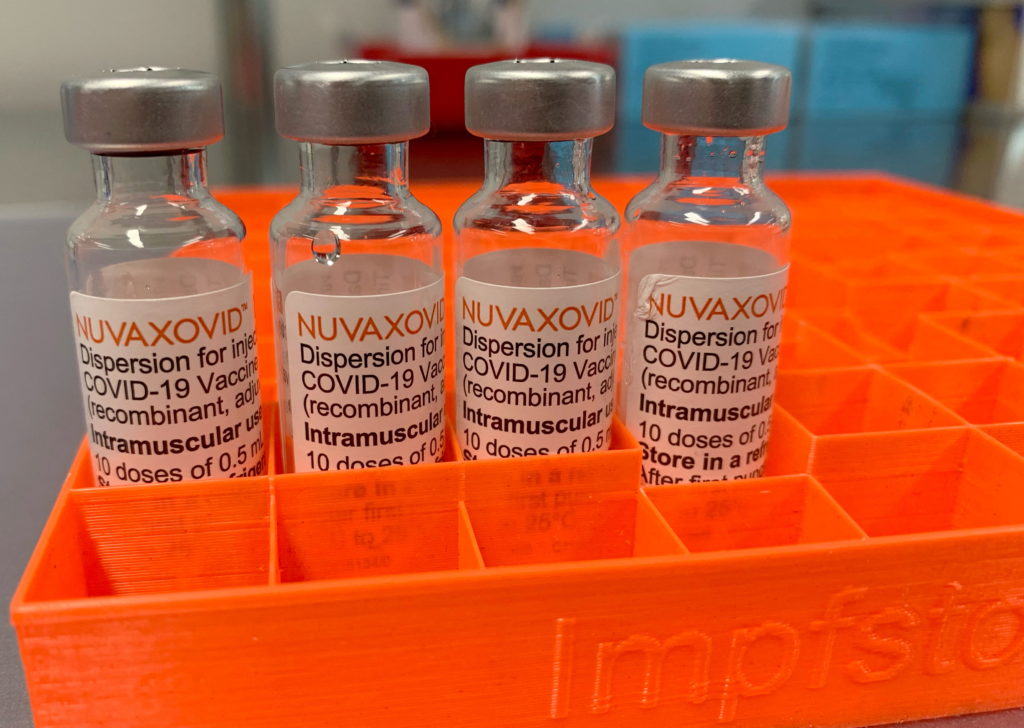
The U.S. Food and Drug Administration (FDA) granted Novavax's COVID-19 vaccine, Nuvaxovid, conditional approval under the Emergency Use Authorization (EUA) in July 2023. This isn't a full licensure; it signifies that the benefits of the vaccine outweigh its known and potential risks under the current emergency situation. This conditional approval differs from the full approval granted to other COVID-19 vaccines like Pfizer-BioNTech and Moderna. The FDA’s decision was based on extensive clinical trial data demonstrating the vaccine's efficacy and safety profile.
Why the Restricted Use?
Despite its approval, the Novavax vaccine's use is restricted, primarily due to factors related to its efficacy and the evolving COVID-19 situation:
-
Lower Efficacy Compared to mRNA Vaccines: Clinical trials showed Nuvaxovid to be less effective against some COVID-19 variants compared to the mRNA vaccines. This doesn't render it ineffective, but it influences its recommended usage.
-
Emergence of New Variants: The constantly evolving nature of the SARS-CoV-2 virus, with new variants emerging regularly, impacts the long-term efficacy of all COVID-19 vaccines, including Novavax. This necessitates ongoing monitoring and potential adjustments to vaccination strategies.
-
Competition in the Market: With other COVID-19 vaccines already widely available, the market demand for a new vaccine with potentially lower efficacy might be limited.
Who Should Consider the Novavax Vaccine?
The restricted use doesn't mean the vaccine is unsuitable. It's particularly targeted towards individuals:
-
Hesitant about mRNA Technology: For those who prefer a protein-based vaccine over mRNA technology, Novavax offers an alternative option. This is crucial for addressing vaccine hesitancy and improving overall vaccination rates.
-
Specific Health Concerns: Certain individuals may have pre-existing conditions or allergies that make them ineligible for mRNA vaccines. Novavax could provide a suitable alternative in these cases. Always consult with your doctor to determine the best vaccine for your individual needs.
Looking Ahead: The Future of Novavax and COVID-19 Vaccination
The conditional approval of the Novavax vaccine is a step forward, offering more options for vaccination. However, ongoing monitoring of its efficacy against emerging variants and its long-term safety profile are critical. The FDA will continue to evaluate the vaccine’s performance and may adjust its recommendations based on new data. The future of COVID-19 vaccination likely involves a multifaceted approach, employing different vaccine technologies to combat the evolving virus.
Keywords: Novavax, COVID-19 vaccine, FDA approval, conditional approval, Emergency Use Authorization (EUA), mRNA vaccine, protein-based vaccine, vaccine hesitancy, COVID-19 variants, vaccine efficacy, Nuvaxovid, vaccination strategy.
Call to Action: Consult your healthcare provider to discuss which COVID-19 vaccine is best suited for your individual needs and health history. Staying informed about COVID-19 updates and vaccination recommendations is crucial for protecting your health and community.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine's Restricted Use. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Rookie Sensation How Shwartzmans Pole Impacts 2025 Indy 500 Betting Odds
May 20, 2025
Rookie Sensation How Shwartzmans Pole Impacts 2025 Indy 500 Betting Odds
May 20, 2025 -
 Over 5 Billion Inflows The Rising Tide Of Bitcoin Etf Investments
May 20, 2025
Over 5 Billion Inflows The Rising Tide Of Bitcoin Etf Investments
May 20, 2025 -
 Jon Rahm Returns To Major Contention The Liv Golf Factor
May 20, 2025
Jon Rahm Returns To Major Contention The Liv Golf Factor
May 20, 2025 -
 Indy 500 2025 Betting Analyzing Shwartzmans Odds After Securing Pole
May 20, 2025
Indy 500 2025 Betting Analyzing Shwartzmans Odds After Securing Pole
May 20, 2025 -
 Jenn Sterger Details Emotional Toll Of Brett Favre Sext Scandal
May 20, 2025
Jenn Sterger Details Emotional Toll Of Brett Favre Sext Scandal
May 20, 2025
