Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Rollout
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Rollout
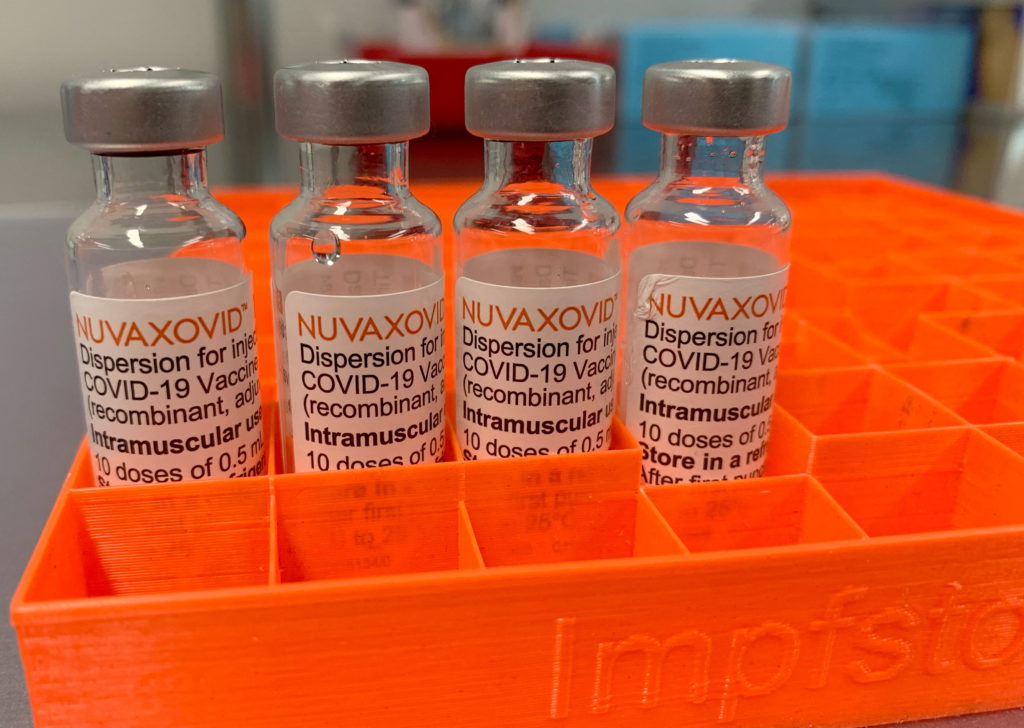
The race for COVID-19 vaccine dominance saw another contender enter the arena this week, as the FDA granted conditional approval to the Novavax COVID-19 vaccine, Nuvaxovid. However, the rollout is far from a full-scale launch, raising questions about its impact and market position. This limited rollout underscores the complexities of vaccine distribution and the evolving landscape of the pandemic.
A Long-Awaited Approval, But With Caveats
Novavax's Nuvaxovid, a protein subunit vaccine, has finally received the green light from the FDA, marking a significant milestone for the company. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, or the viral vector vaccines such as Johnson & Johnson's Janssen, Nuvaxovid utilizes a more traditional approach. This traditional technology might appeal to some vaccine hesitant individuals who harbor concerns about the newer mRNA technology. The conditional approval, however, comes with limitations. The initial rollout will be significantly smaller than the initial launches of other COVID-19 vaccines, targeting specific demographics and regions.
Why the Limited Rollout?
Several factors contribute to the limited initial rollout of the Novavax vaccine:
- Demand: With other vaccines widely available and booster campaigns underway, the current demand for a new vaccine is relatively low. Many individuals have already received other COVID-19 vaccinations.
- Manufacturing Capacity: Scaling up vaccine production is a complex and time-consuming process. Novavax may need more time to ramp up production to meet a larger-scale demand.
- Regulatory Hurdles: Securing regulatory approvals in different countries can be a lengthy process, further delaying widespread distribution.
- Strategic Allocation: The initial supply may be strategically allocated to specific populations or regions where other vaccines may have had lower uptake.
Who Will Receive the Vaccine First?
While specifics are still emerging, the initial distribution strategy may prioritize groups who have expressed hesitancy towards mRNA vaccines or who might benefit from having an alternative option. This could include certain age groups or specific communities. The Centers for Disease Control and Prevention (CDC) will play a crucial role in advising on the allocation strategy.
The Future of Novavax's Vaccine
The conditional approval is undoubtedly a positive step for Novavax. However, the limited rollout presents challenges. The company needs to effectively communicate the vaccine's benefits and address any remaining public concerns about its safety and efficacy. Success will depend on:
- Effective Public Health Messaging: Clear and concise communication about the vaccine's efficacy and safety profile is essential to encourage uptake.
- Efficient Distribution Networks: Collaborating with healthcare providers and distribution networks is crucial to ensure equitable access.
- Ongoing Monitoring and Data Collection: Continuous monitoring of the vaccine's effectiveness and safety post-market release is critical.
The success of Novavax's COVID-19 vaccine remains to be seen. While the conditional FDA approval is a victory, the road ahead involves navigating logistical challenges, addressing public perception, and demonstrating its long-term impact on global public health. The coming months will provide crucial insights into its role in the ongoing fight against COVID-19.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, vaccine rollout, protein subunit vaccine, mRNA vaccine, vaccine hesitancy, public health, vaccine distribution, CDC.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Rollout. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Nfl Rookie Life The First Months Reality Check Espn
May 21, 2025
Nfl Rookie Life The First Months Reality Check Espn
May 21, 2025 -
 Rain And Cold To Impact Region Throughout Wednesday
May 21, 2025
Rain And Cold To Impact Region Throughout Wednesday
May 21, 2025 -
 Sotos Lack Of Hustle Mets To Hold Meeting After Hr Turned Single
May 21, 2025
Sotos Lack Of Hustle Mets To Hold Meeting After Hr Turned Single
May 21, 2025 -
 Pga Championship Heartbreak Jon Rahms Resilience In Defeat
May 21, 2025
Pga Championship Heartbreak Jon Rahms Resilience In Defeat
May 21, 2025 -
 Animal Welfare In Assassins Creed Valhalla Ubisofts Design Choices
May 21, 2025
Animal Welfare In Assassins Creed Valhalla Ubisofts Design Choices
May 21, 2025
