Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions
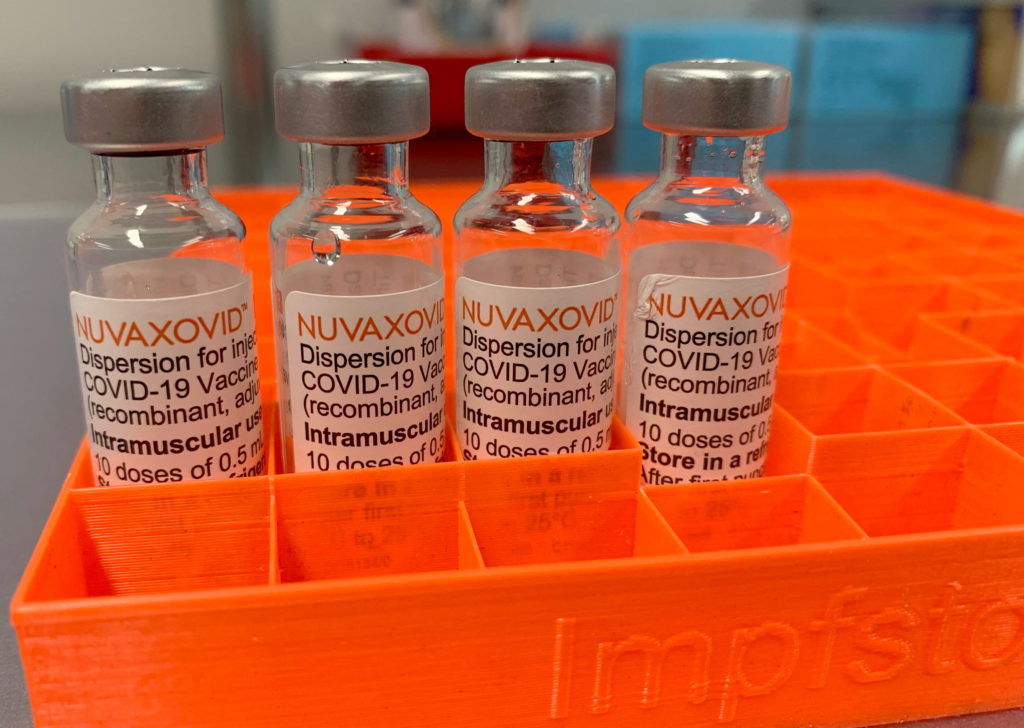
The FDA's conditional approval of the Novavax COVID-19 vaccine, Nuvaxovid, marks a significant step in the fight against the pandemic, but it's not without its caveats. While offering another option for individuals hesitant about mRNA vaccines, the approval comes with specific usage restrictions, limiting its immediate impact and raising questions about its future role in vaccination campaigns.
Limited Authorization, Specific Populations: Unlike the widely used Pfizer-BioNTech and Moderna vaccines, the FDA's authorization for Nuvaxovid is more targeted. The vaccine is currently approved only for individuals 18 years of age and older, a key limitation considering the broader age range covered by other COVID-19 vaccines. This restricted authorization stems from a more limited data set compared to its mRNA counterparts, particularly regarding efficacy and safety in younger populations. Further studies are needed to assess its effectiveness and safety profile in younger age groups.
Why the Conditional Approval? The conditional approval signifies that the FDA has found the vaccine to be safe and effective based on available data, but further studies are needed to confirm its long-term effects and efficacy against emerging variants. This approach allows for quicker access to the vaccine while simultaneously requiring ongoing monitoring and data collection. This differs from the full approvals granted to other COVID-19 vaccines, which have benefited from significantly larger and longer-term clinical trials.
Understanding the Nuvaxovid Technology: Unlike the mRNA vaccines, Nuvaxovid uses a more traditional protein subunit technology. This technology involves using harmless pieces of the virus to trigger an immune response. Many people find this approach more familiar and less concerning than the relatively newer mRNA technology. This technological difference is a key factor driving interest in the Novavax vaccine, offering an alternative for those wary of mRNA-based vaccines. For more information on vaccine technology, you can visit the .
Efficacy and Safety Concerns: While the FDA deemed Nuvaxovid safe and effective, its efficacy rate is notably lower than the mRNA vaccines in clinical trials. While this doesn't necessarily render it ineffective, it does highlight a potential for less robust protection compared to other available options. Further research is crucial to understand the nuances of its efficacy against different variants and long-term immunity. The FDA will continue to monitor safety data post-authorization to identify and address any potential issues.
Future Implications and Market Position: The conditional approval, coupled with the usage restrictions, creates an interesting scenario for Novavax's market position. While it provides an alternative vaccine option, its limited authorization may impact its overall uptake. The vaccine's success will largely depend on ongoing research, addressing concerns about efficacy, and expanding its authorized usage to a wider population. The long-term effectiveness against emerging variants also remains a crucial factor in determining its future role in global vaccination strategies.
Looking Ahead: The Novavax COVID-19 vaccine represents a valuable addition to the global vaccine arsenal, but its future success hinges on further research and addressing the current limitations. The conditional approval underscores the ongoing evolution of the COVID-19 vaccine landscape and highlights the importance of continued vigilance and scientific research in managing this evolving pandemic. Stay informed about updates from the FDA and CDC regarding the ongoing evaluation and potential expansion of Nuvaxovid's usage.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Trumps Diminished Role Putins Actions Speak Volumes
May 20, 2025
Trumps Diminished Role Putins Actions Speak Volumes
May 20, 2025 -
 Indy 500 Practice Examining A Series Of Accidents
May 20, 2025
Indy 500 Practice Examining A Series Of Accidents
May 20, 2025 -
 Confirmed A New Peaky Blinders Series Is Coming Expect The Unexpected
May 20, 2025
Confirmed A New Peaky Blinders Series Is Coming Expect The Unexpected
May 20, 2025 -
 Win Big Your Guide To The Best Mlb Bets On May 19 2025
May 20, 2025
Win Big Your Guide To The Best Mlb Bets On May 19 2025
May 20, 2025 -
 New Peaky Blinders Series Confirmed What We Know About The Big Change
May 20, 2025
New Peaky Blinders Series Confirmed What We Know About The Big Change
May 20, 2025
