Conditional FDA Approval For Novavax COVID-19 Vaccine: What You Need To Know
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
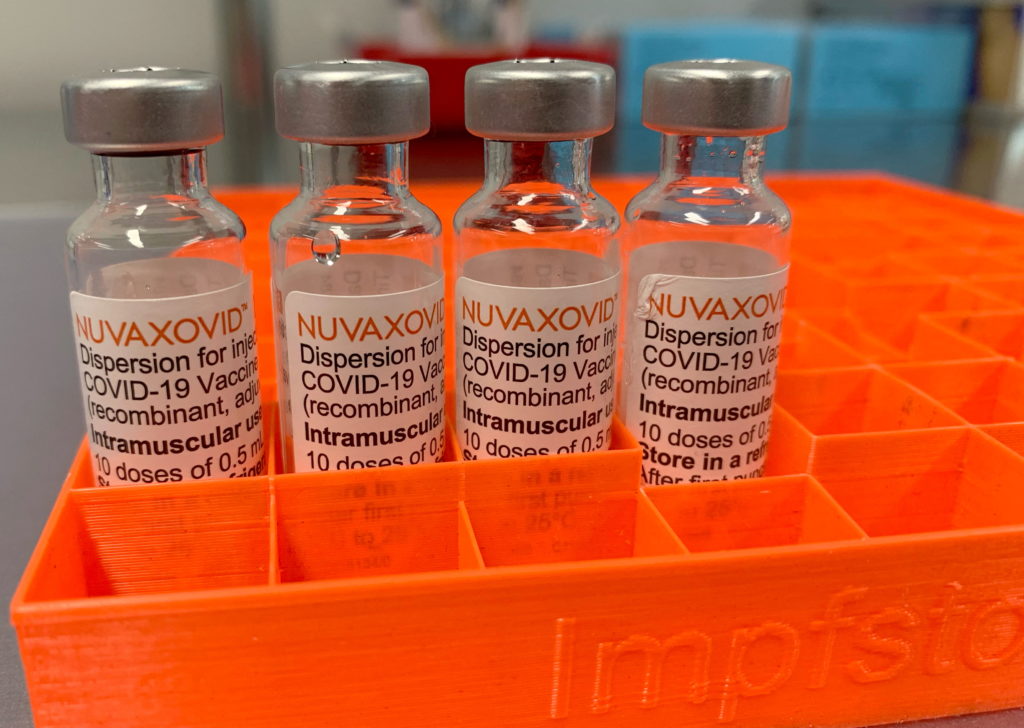
Conditional FDA Approval for Novavax COVID-19 Vaccine: What You Need to Know
The race for effective COVID-19 vaccines saw another significant development with the Novavax vaccine receiving Conditional FDA approval. This news offers a new option for individuals hesitant about mRNA vaccines, but understanding the specifics is crucial. This article will delve into the key aspects of this approval, addressing efficacy, safety, and what it means for the ongoing fight against the pandemic.
What is the Novavax COVID-19 Vaccine?
Unlike the Pfizer-BioNTech and Moderna vaccines which utilize mRNA technology, the Novavax vaccine uses a more traditional approach. It's a protein-subunit vaccine, meaning it uses harmless pieces of the virus's protein (spike protein) to trigger an immune response. This method has been used for decades in vaccines against other diseases like hepatitis B and influenza. Many individuals find this approach more familiar and reassuring, potentially increasing vaccine uptake among hesitant populations.
Efficacy and Safety Data:
The FDA's conditional approval was based on extensive clinical trials. Data showed the Novavax vaccine to be highly effective in preventing COVID-19, particularly against severe illness and hospitalization. While the exact efficacy numbers may vary depending on the specific study and the prevailing COVID-19 variant, the overall results demonstrate a strong protective effect. Importantly, the safety profile of the Novavax vaccine appears comparable to other authorized COVID-19 vaccines, with common side effects generally being mild and temporary, such as injection site pain, fatigue, and headache. However, as with all vaccines, rare but serious side effects are possible. Detailed information on reported side effects can be found on the .
Who Should Get the Novavax Vaccine?
The Novavax vaccine is authorized for use in individuals 18 years of age and older. While it offers a valuable alternative for those who prefer a non-mRNA vaccine, it's important to consult with a healthcare provider to discuss individual health needs and determine the most suitable vaccine. This is especially important for individuals with pre-existing medical conditions or allergies.
The Significance of Conditional Approval:
The term "conditional" approval highlights that the FDA's approval is based on ongoing data collection and analysis. The manufacturer, Novavax, is required to continue monitoring the vaccine's safety and efficacy after its authorization. This ongoing surveillance allows for a rapid response if any unexpected issues arise. This is a standard procedure for many newly approved medications and vaccines.
Addressing Vaccine Hesitancy:
The Novavax vaccine's approval could play a significant role in addressing vaccine hesitancy. For individuals who have been reluctant to receive mRNA vaccines due to concerns about the technology's novelty, the familiar protein-subunit approach may offer a more comfortable option. This broadened availability could contribute to higher overall vaccination rates, furthering the progress toward herd immunity and mitigating the impact of future COVID-19 outbreaks.
Looking Ahead:
The conditional FDA approval of the Novavax COVID-19 vaccine is a positive step in the global fight against the pandemic. While the long-term effects of the vaccine are still being studied, the available data suggests a safe and effective addition to the existing arsenal of COVID-19 vaccines. This offers increased choice and potentially a path to higher vaccination rates, leading to better public health outcomes. Stay informed by consulting your doctor and reliable sources like the CDC and FDA websites for the latest updates on COVID-19 vaccines and their efficacy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval For Novavax COVID-19 Vaccine: What You Need To Know. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Mira El Dueto De Yahir Y Victor Garcia Otra Vez En Juego De Voces 2025
May 20, 2025
Mira El Dueto De Yahir Y Victor Garcia Otra Vez En Juego De Voces 2025
May 20, 2025 -
 Carlos Correa And Byron Buxton Twins Stars Hit The Il With Concussions
May 20, 2025
Carlos Correa And Byron Buxton Twins Stars Hit The Il With Concussions
May 20, 2025 -
 Unbelievable Numbers Unveiling The Top Stats From The 2023 College Football Season
May 20, 2025
Unbelievable Numbers Unveiling The Top Stats From The 2023 College Football Season
May 20, 2025 -
 Rafael Devers Red Soxs Designated Hitter Finds Success
May 20, 2025
Rafael Devers Red Soxs Designated Hitter Finds Success
May 20, 2025 -
 Game 7 Rout How The Oklahoma City Thunder Conquered The Denver Nuggets
May 20, 2025
Game 7 Rout How The Oklahoma City Thunder Conquered The Denver Nuggets
May 20, 2025
