Conditional FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval for Novavax COVID-19 Vaccine: Understanding the Restrictions
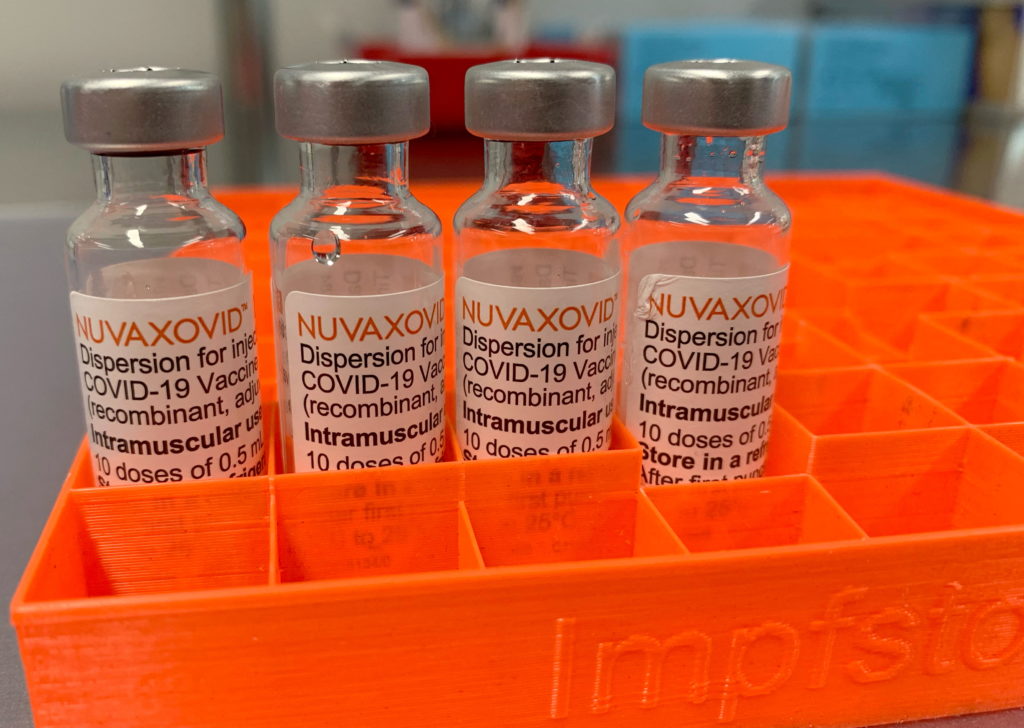
The Novavax COVID-19 vaccine, Nuvaxovid, has received conditional FDA approval, marking a significant development in the fight against the pandemic. However, this approval comes with specific restrictions. Understanding these limitations is crucial for both healthcare providers and the public to make informed decisions about vaccination. This article will delve into the details of the conditional approval and explore the implications for vaccine rollout and efficacy.
What Does Conditional FDA Approval Mean?
Unlike a full FDA approval, conditional approval signifies that the agency has assessed the vaccine's benefits to outweigh its risks based on available data, but further studies and long-term safety monitoring are still required. This approach allows quicker access to potentially life-saving medical products during public health emergencies, such as the COVID-19 pandemic. However, the FDA retains the authority to revoke or modify the approval if new safety concerns or efficacy data emerge. This is a key difference between conditional approval and full licensure.
Nuvaxovid: A Different Approach to COVID-19 Vaccination
Novavax's vaccine uses a different technology than the mRNA vaccines (Pfizer-BioNTech and Moderna) and the viral vector vaccines (Johnson & Johnson). It's a protein subunit vaccine, utilizing a lab-made version of the spike protein found on the surface of the SARS-CoV-2 virus. This approach may appeal to individuals hesitant about mRNA or viral vector technologies. The protein subunit approach has been used for decades in other vaccines, potentially building public trust. However, it's important to note that all COVID-19 vaccines have undergone rigorous testing and have proven effective in preventing severe illness, hospitalization, and death.
Restrictions and Limitations of the Conditional Approval:
The conditional approval of Nuvaxovid is subject to several restrictions. These include:
- Ongoing Surveillance: The FDA mandates continuous monitoring of the vaccine's safety and effectiveness through post-market surveillance. This involves tracking adverse events and evaluating long-term efficacy against emerging variants.
- Specific Age Groups: The initial approval may be limited to specific age groups, depending on the available clinical trial data. Further studies might be needed to expand approval to other age demographics.
- Manufacturing Requirements: The FDA will likely impose strict requirements on the manufacturing process to ensure consistent quality and safety of the vaccine batches.
- Labeling Requirements: Clear and comprehensive labeling is crucial to inform healthcare providers and recipients about the vaccine's benefits, risks, and limitations. This includes potential side effects and contraindications.
What This Means for You:
The conditional approval of the Novavax vaccine expands the options available for individuals seeking COVID-19 vaccination. It offers a different technological approach for those who prefer a protein subunit vaccine. However, it’s crucial to remember that all authorized COVID-19 vaccines provide significant protection against severe illness. Consult your healthcare provider to determine which vaccine is best suited to your individual health circumstances and risk profile.
Looking Ahead:
The ongoing monitoring and further research on Nuvaxovid will be crucial in fully understanding its long-term safety and effectiveness. The conditional approval represents a step forward in the global fight against COVID-19, providing an additional tool in our arsenal to protect public health. Further updates from the FDA are expected as more data becomes available. Stay informed about the latest developments through reliable sources like the and the .
Call to Action: Talk to your doctor about COVID-19 vaccination and which vaccine is right for you. Remember that vaccination remains a critical tool in protecting yourself and your community.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 S And P 500 Dow Nasdaq Climb Six Day Winning Streak Continues Amidst Moodys Action
May 20, 2025
S And P 500 Dow Nasdaq Climb Six Day Winning Streak Continues Amidst Moodys Action
May 20, 2025 -
 Jets Loss Overshadowed By Scheifele Family Tragedy
May 20, 2025
Jets Loss Overshadowed By Scheifele Family Tragedy
May 20, 2025 -
 Espns A J Perez Recounts Threats From Brett Favres Camp
May 20, 2025
Espns A J Perez Recounts Threats From Brett Favres Camp
May 20, 2025 -
 Knicks Fans Roasted Thunder Fans Praised Trae Youngs Post Game Comments Spark Debate
May 20, 2025
Knicks Fans Roasted Thunder Fans Praised Trae Youngs Post Game Comments Spark Debate
May 20, 2025 -
 Balis Tourism Overhaul Stricter Guidelines To Combat Negative Tourist Impacts
May 20, 2025
Balis Tourism Overhaul Stricter Guidelines To Combat Negative Tourist Impacts
May 20, 2025
