Conditional Approval: FDA Authorizes Novavax COVID-19 Vaccine With Specific Guidelines
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
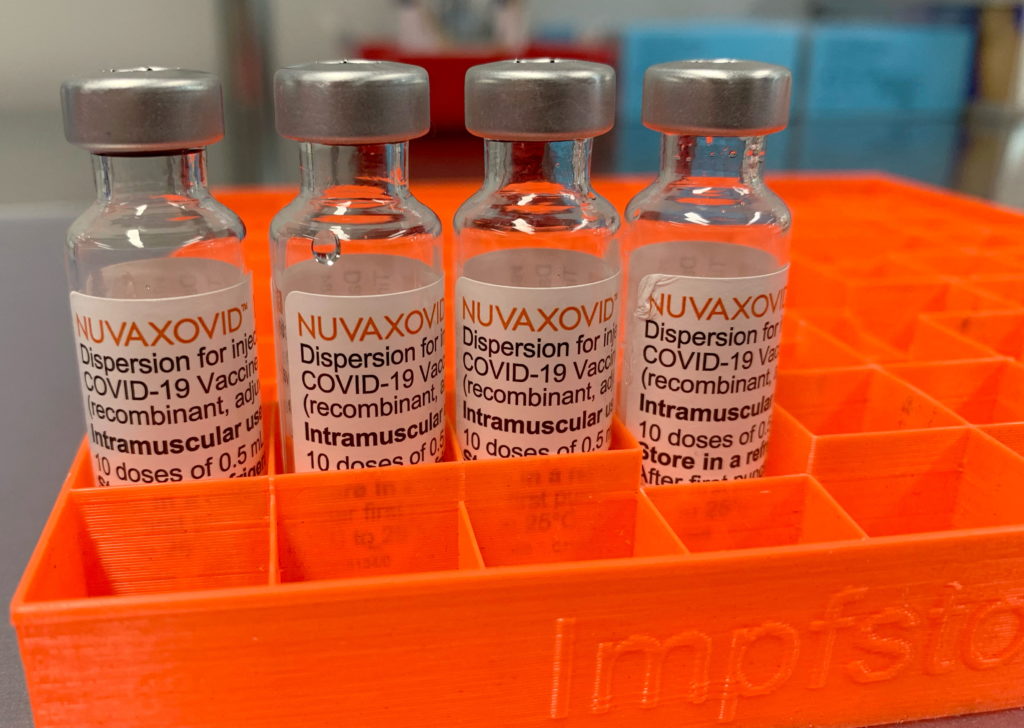
Conditional Approval: FDA Authorizes Novavax COVID-19 Vaccine with Specific Guidelines
The FDA's conditional approval of the Novavax COVID-19 vaccine marks a significant development in the fight against the pandemic, offering an alternative option for those hesitant to receive mRNA vaccines. However, this authorization comes with specific guidelines and considerations that individuals and healthcare providers should understand.
A New Contender in the COVID-19 Vaccine Landscape
The Novavax vaccine, marketed under the name Nuvaxovid, uses a different technology compared to the Pfizer-BioNTech and Moderna vaccines. Instead of mRNA, it employs a protein subunit technology, a more traditional approach to vaccine development. This protein-based approach may appeal to individuals concerned about the newer mRNA technology. The FDA's approval is conditional, meaning it's granted based on ongoing data collection and analysis. This approach allows for quicker access to potentially life-saving treatments while continuing to monitor long-term safety and efficacy.
Understanding the FDA's Conditional Approval and its Implications
The conditional approval is not without its stipulations. The FDA has outlined specific guidelines for administration, monitoring, and reporting adverse events. This rigorous oversight ensures the vaccine's continued safety and efficacy are closely monitored. The conditional nature of the approval reflects the FDA's commitment to a data-driven approach to vaccine authorization. This allows for flexibility in response to new data or emerging concerns.
Key Aspects of the Novavax Vaccine and its Approval:
- Technology: Nuvaxovid uses a protein subunit technology, which involves using specific pieces of the virus to trigger an immune response. This differs from the mRNA vaccines that use genetic material to instruct cells to produce the viral protein.
- Two-Dose Regimen: The vaccine is administered as a two-dose primary series, given three to eight weeks apart.
- Efficacy: Clinical trials demonstrated high efficacy against COVID-19, particularly against severe disease. The specific efficacy rates vary depending on the trial and variant under consideration.
- Safety Profile: While generally well-tolerated, the FDA requires continued monitoring of potential side effects, similar to other COVID-19 vaccines. Common side effects reported include pain at the injection site, fatigue, headache, muscle aches, and nausea. Serious side effects, while rare, should be reported immediately to healthcare providers.
- Target Population: Currently, the vaccine is authorized for individuals 18 years of age and older. Further studies may lead to expansion of the authorized age range.
Who Might Benefit from the Novavax Vaccine?
The Novavax vaccine provides a valuable option for individuals who:
- Have concerns about mRNA vaccine technology.
- Prefer a more traditional vaccine approach.
- May have experienced adverse reactions to other COVID-19 vaccines.
Finding the Novavax Vaccine and Important Considerations:
It's important to consult with your healthcare provider to determine if the Novavax vaccine is appropriate for you. They can discuss your individual medical history and risk factors to help you make an informed decision. The availability of the Novavax vaccine will vary by location, so checking with local pharmacies and healthcare providers is recommended.
Looking Ahead: Continued Monitoring and Research
The FDA's conditional approval is a significant step, but it's crucial to remember that ongoing monitoring and research are essential. The FDA continues to collect data to fully understand the long-term safety and efficacy of the Novavax vaccine. This commitment to ongoing surveillance reassures the public that the safety and effectiveness of the vaccine remain a top priority. Further research is also underway to assess the vaccine’s effectiveness against emerging COVID-19 variants.
This ongoing research and data analysis will ensure that healthcare professionals and the public have the most up-to-date information regarding the Novavax vaccine's safety and efficacy. The FDA’s commitment to transparency and data-driven decision-making remains a cornerstone of public health efforts during this ongoing pandemic.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional Approval: FDA Authorizes Novavax COVID-19 Vaccine With Specific Guidelines. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 David Adelmans Coaching Style Winning Over Players And Critics In Denver
May 21, 2025
David Adelmans Coaching Style Winning Over Players And Critics In Denver
May 21, 2025 -
 Fan Fury Jon Jones Latest Comments On Tom Aspinall Ignite Social Media
May 21, 2025
Fan Fury Jon Jones Latest Comments On Tom Aspinall Ignite Social Media
May 21, 2025 -
 Solo Leveling Garners Prestigious Award More Awards Expected
May 21, 2025
Solo Leveling Garners Prestigious Award More Awards Expected
May 21, 2025 -
 Wyndham Clark Faces Backlash After Pga Championship Driver Incident
May 21, 2025
Wyndham Clark Faces Backlash After Pga Championship Driver Incident
May 21, 2025 -
 Can The Boston Celtics Sustain Their Championship Push
May 21, 2025
Can The Boston Celtics Sustain Their Championship Push
May 21, 2025
